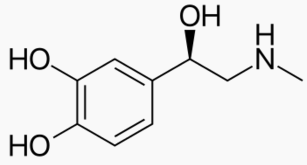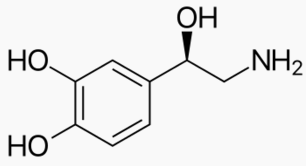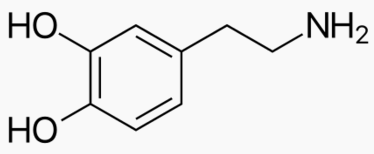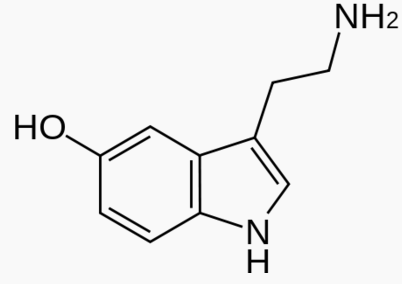
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
|---|
 |
|---|
 |
 |
|---|
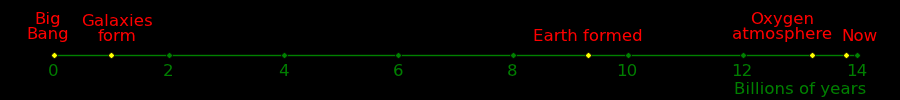
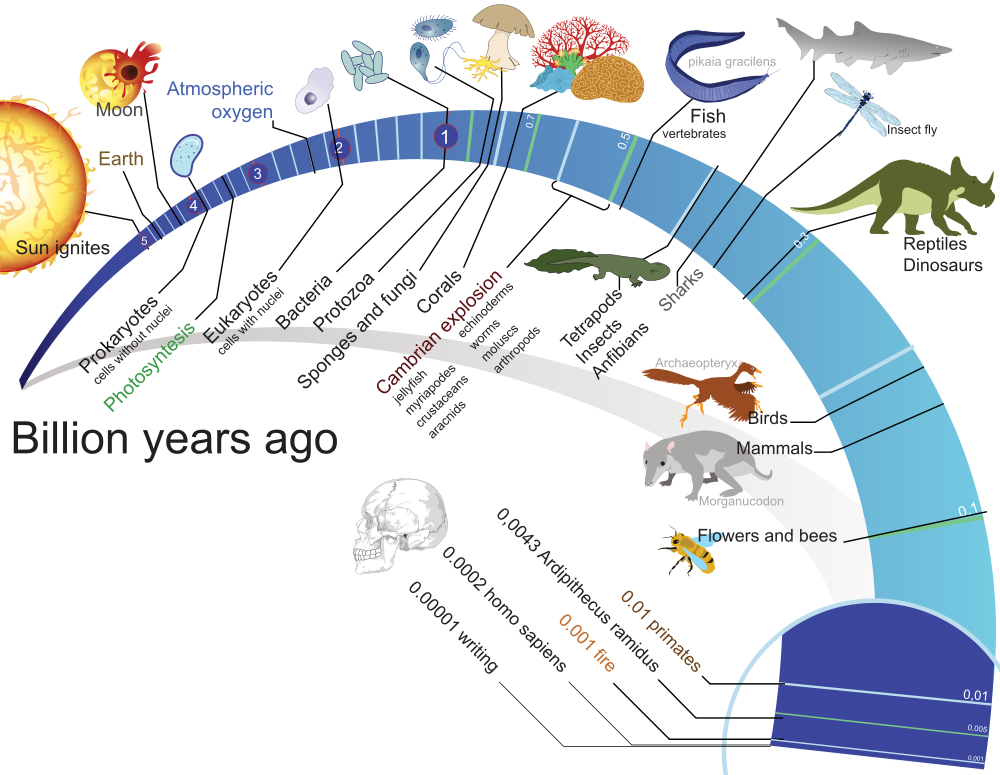
Millions of years ago
Big bang 13700
First planets formed 13000
Earth formed 4500
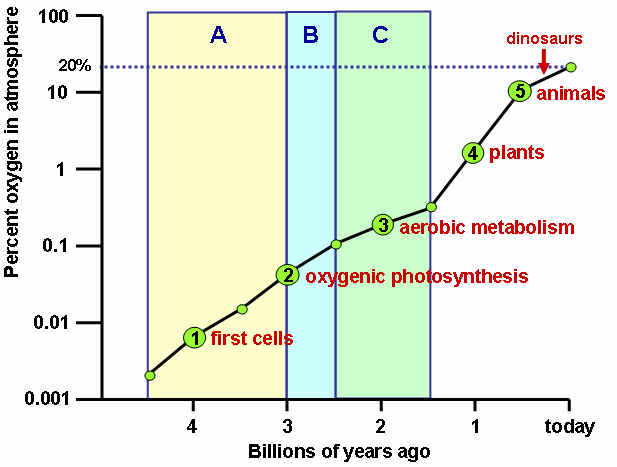
Photosynthesis 3000
Oxygen atmosphere 600
Multicellular life 600
Vertebrates 480
Tetrapod vertebrates 400
Mammals 170
Dinosaur extinction 66
Cats 25
Cheetahs 6
Tigers 1.8
Humans 1
Lions .9
Agriculture .01
Civilization .005
Calculus .0004
An alien planet could conceivably have formed as early as 1 billion years after the
big bang, meaning that there are likely aliens with a head start on us by billions of
years.
An alien civilization could easily build a rocket that travels at 1/10 the speed of light, which would take 1 million years to cross the galaxy. The aliens have plenty of time to get here.
 |
 |
|---|---|
 |
|---|
 |
 |
|---|---|
 |
|---|
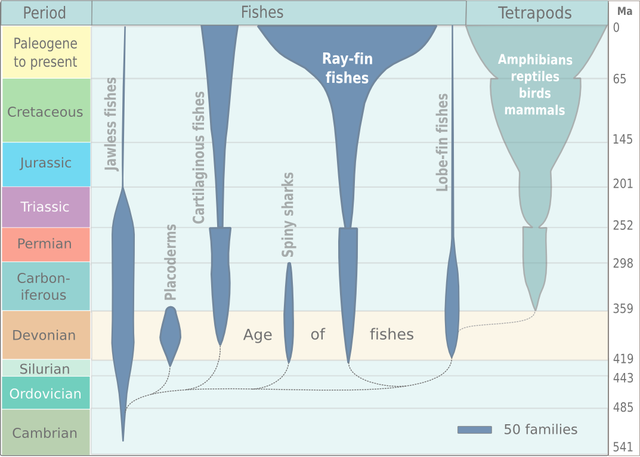
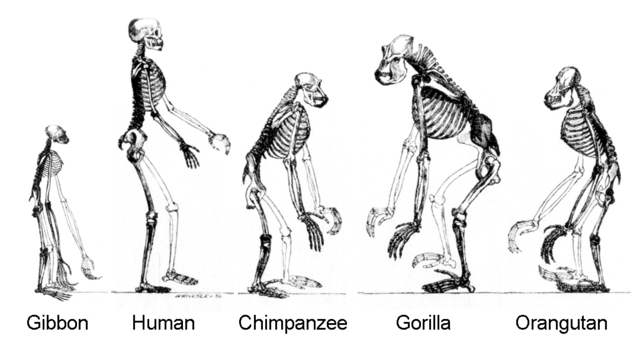
-> Reptiles -> Dinosaurs -> Birds
/
Vertebrates -> Tetrapod vertebrates --
\
-> Mammals
A tetrapod is a vertebrate with four limbs. Reptiles, dinosaurs, birds and
mammals all evolved from tetrapods, and the essential elements of the tetrapod
design haven't changed since its emergence. Elements of the tetrapod design
include:
A spine
A skull
A ribcage
Four limbs
One bone in the upper limb and two bones in the lower limb.
Humans have the most complex wrists and hands in the animal kingdom.
Bruce Lee: There is only one type of body, 2 arms, 2 legs, etc that make up the human body. Therefore, there can only be one style of fighting. If the other guy had 4 arms and 2 legs, there might have to be a different one. Forget the belief that one style is better than the other, the point of someone that does not just believe in tradition, but actually wants to know how to fight is to take what you need from every martial art and incorporate it into your own. Make it effective and very powerful, but don't worry if you are taking moves from many different arts, that is a good thing.
 |
 |
 |
|---|---|---|
 |
 |
 |
|---|---|---|
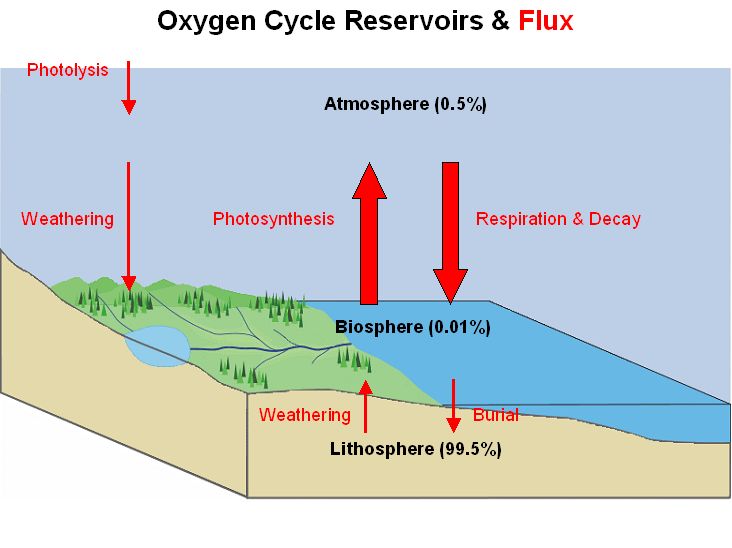
Define one "Earth Atmosphere Oxygen Mass" (EAOM) as the mass of the oxygen in the Earth's atmosphere, equal to 1.4*10^18 kg.
Oxygen Mass Flux Half life Reservoir (EAOM) (EAOM/year) Atmosphere 1 .000214 4500 years Biosphere .0114 .000214 50 years Lithosphere 207 4.3*10^-7 400 million years Land photosynthesis = .000118 EAOM/year = 165*10^12 kg Oxygen / year Ocean photosynthesis = .000096 EAOM/year = 135*10^12 kg Oxygen / yearThe time required by photosynthesis to generate one EAOM is 4700 years.
Early in the Earth's history, oxygen produced by photosynthesis was absorbed by iron dissolved in the oceans, creating "banded iron formations". Once photosynthesis overwhelmed the ocean's iron, an oxygen atmosphere became possible.
 |
 |
|---|---|
A desert planet like Tatooine would have a hard time generating an oxygen atmosphere.
 |
|---|
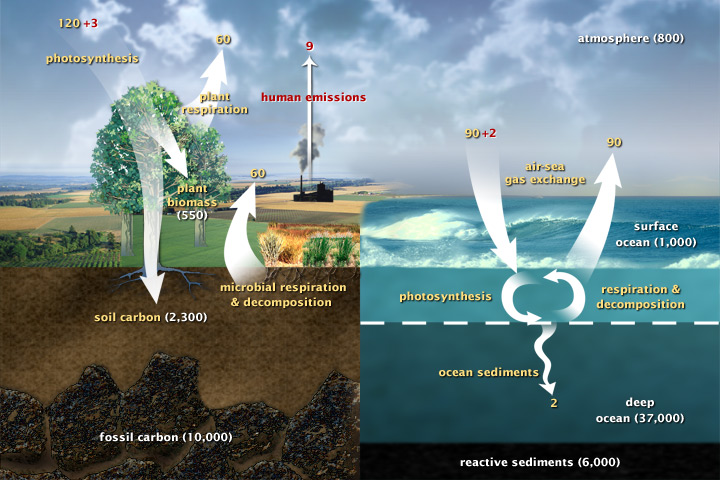
This diagram of the fast carbon cycle shows the motion of carbon between land, atmosphere, and oceans, in billions of tons of carbon per year. Yellow numbers are natural fluxes, red are human contributions in billions of tons of carbon per year. White numbers indicate stored carbon.
Carbon content of the Earth, with the atmosphere normalized to 1
Atmosphere 1.00 Biomass .62 Surface ocean 1.25 Deep ocean 46.2 Ocean sediment 7.5 Soil 2.88 Fossil carbon 12.5Given the global rate of fossil fuel burning, it would take ~ 89 years to double the atmosphere's carbon.
Photosynthesis moves carbon from the atmosphere to plants, and the carbon returns to the atmosphere through plant respiration and through microbial decomposition of dead plants. Given the global rate of photosynthesis, it takes photosynthesis ~ 7 years to cycle through the atmosphere's carbon.
 |
 |
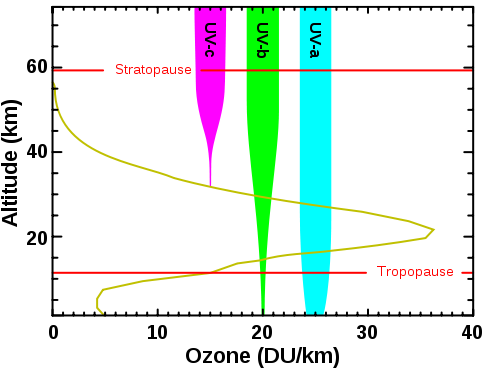 |
|---|---|---|
Ozone production from solar radiation = 400 million tons / day Ozone loss from combining with oxygen radicals = 400 million tons / day Total atmospheric ozone = 3000 million tonsThe Sun produces 12% of the ozone layer each day.
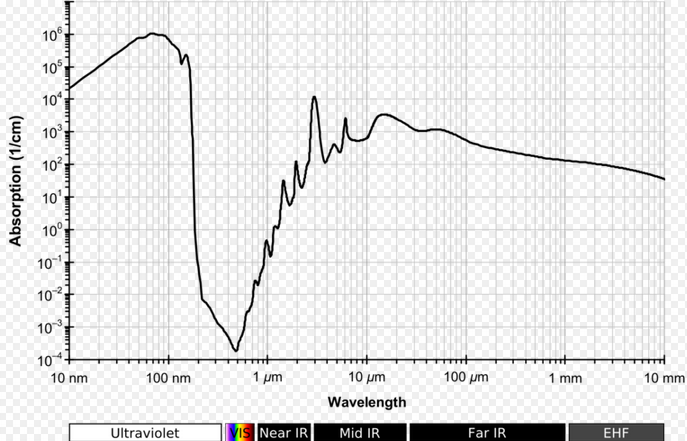 |
|---|
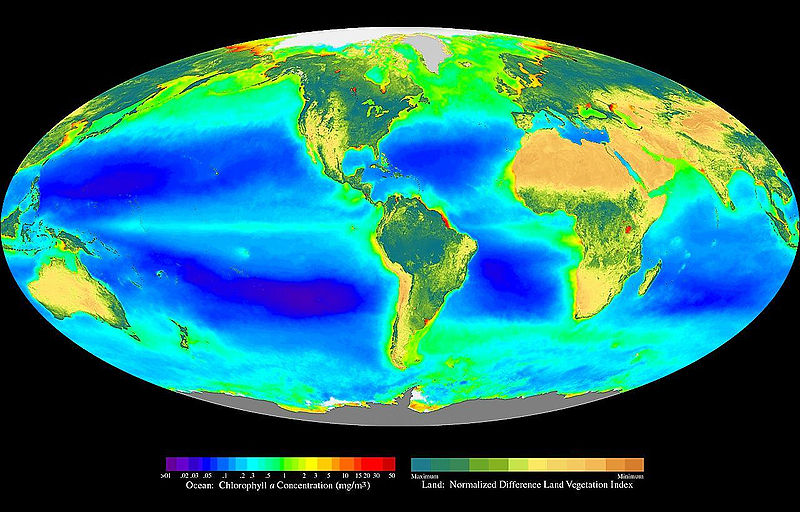
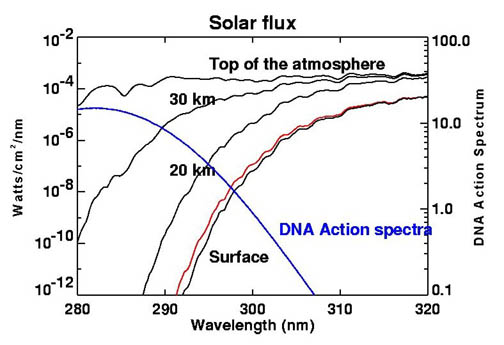
Before the Earth had oxygen and ozone, the continents were uninhabitable and the only place photosynthesis could take place was underwater. Water is more transparent to visible light than to ultraviolet light.
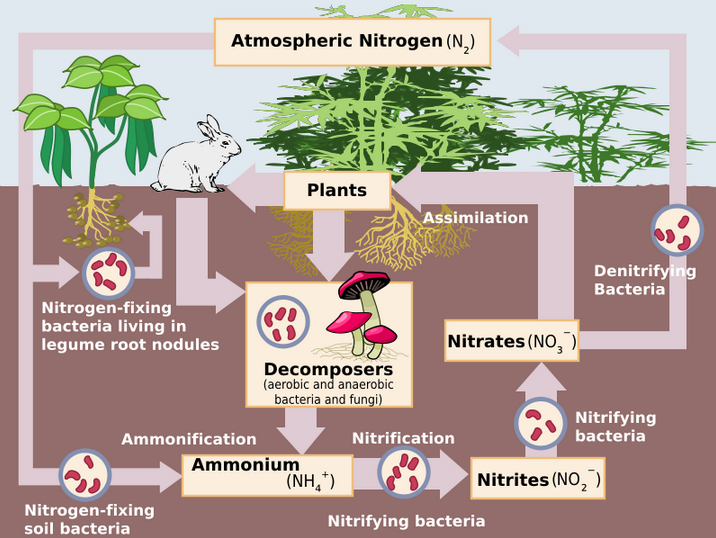 |
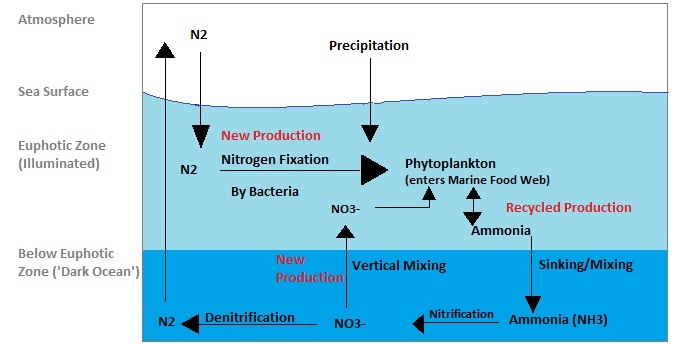 |
|---|---|
Cyanobacteria can fix 1.8 kg of nitrogen per hectare per day. Nitrogen can be fixed in the ocean at twice the rate than what is possible on land.
 |
 |
 |
|---|---|---|
 |
 |
|---|
 |
 |
 |
 |
|---|---|---|---|
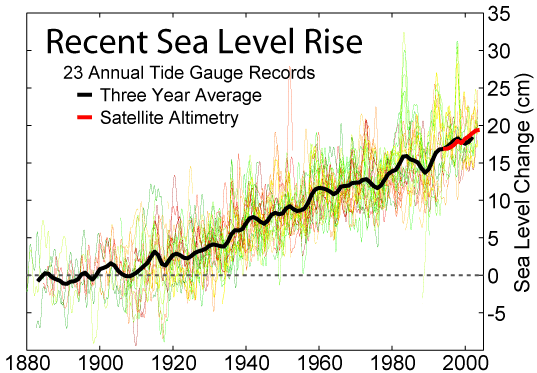
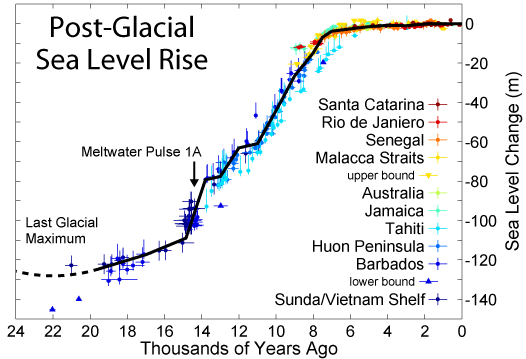
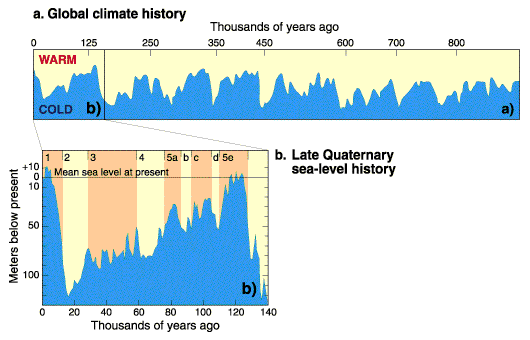
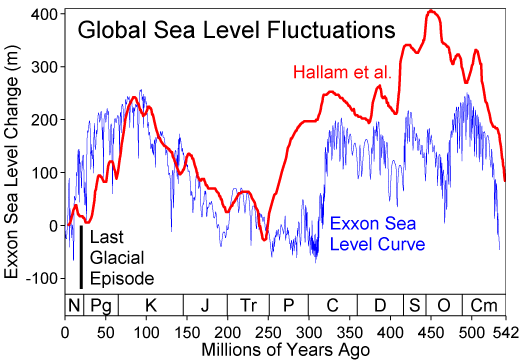
Atmosphere temperature rise= .017 Kelvin/year (.9 Kelvin since 1800) Sea level rise = 2.8 mm/year (225 mm since 1800) Atmosphere CO2 frac = .0035 (.0027 in 1800) Atmosphere carbon =720 Gtons Photosynthesis of carbon =120 Gtons/year Human carbon emissions = 9 Gtons/year = 1240 kg/person/year Energy produced = .57 ZJoules/year = 78.6 GJoules/person/year Electricity produced = .067 ZJoules/year = 9.2 GJoules/person/year Food = .027 ZJoules/year = 3.7 GJoules/person/year = 2500 Cal/person/day Sunlight energy =3850 ZJoules/year Wind energy = 2.25 ZJoules/year Photosynthesis of biomass = 3.00 ZJoules/year Ocean heat gain = 7.5 ZJoules/year World power = 18 TWatts = 4500 Watts/person Energy cost = 16 T$/year = 2210 $/person/year (27.8 $/GJoule) Population = 7.254 billion Food = 1.58 Tkg/year = 218 kg/person/year (As carbs) Earth land area =148.9 Mkm2 = 2.0 Hectares/person Rainfall over land =107000 km3/year = 14.8 tons/person/year River flow = 37300 km3/year = 5140 tons/person/year Water total use = 9700 km3/year = 1390 tons/person/year Water for agriculture = 1526 km3/year = 218 tons/person/year Water for home use = 776 km3/year = 111 tons/person/year Water desalinated = 36 km3/year = 5 tons/person/year
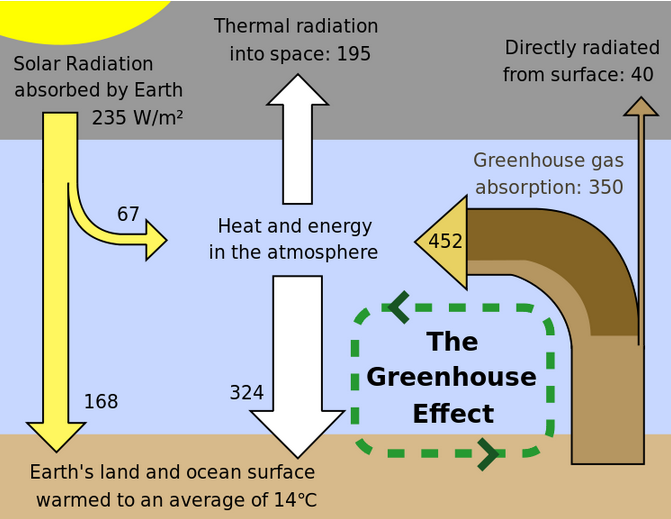 |
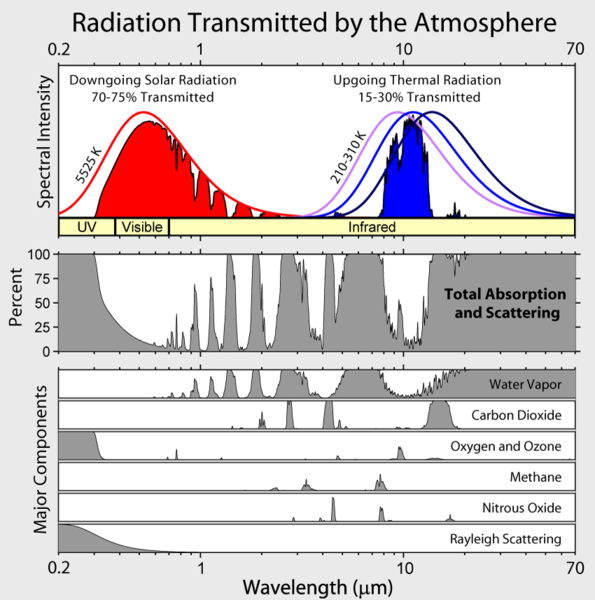 |
|---|---|
Contribution to Half life in the atmosphere
greenhouse warming
H2O 36-72%
CO2 9-26% 90 years
CH4 4-9% 12 years
Ozone 3-7% 1 week
 |
 |
|---|---|
Chuck Sulfur-reducing bacteria
(Oxygen Clan) (Sulfur Clan)
Mass 100 kg single-cellular
Power source Hydrocarbons + oxygen Hydrocarbons + sulfur
ATP per glucose 30 2
Resting power 100 Watts tiny
Peak power kilowatts tiny
Peak power/weight 10 Watts/kg tiny
Strength Kilonewtons tiny
Computation 10^14 synapses -
Brain power 20 Watts -
Achilles heel H2S Oxygen
Aerobic organisms have an energy advantage over anaerobic organisms.
Aerobic respiration: Glucose + Oxygen --> H2O + 30 ATP of energy Anaerobic respiration: Glucose + Sulfur --> H2S + 2 ATP of energyAerobic organisms also have a weight advantage over anaerobic organisms. Aerobic organisms can get oxygen from the air whereas anaerobic organisms have to carry their oxidizer.
For the reaction Hydrocarbons + Oxygen --> H2O + CO2 + energy Mass of oxygen / Mass of hydrocarbons ~ 8These two factors give aerobic organisms an overwhelming energy advantage over anaerobic ones, and this is the reason why nearly all multi-cellular organisms are aerobic.
Oxygen is usually toxic to anaerobic organisms.
Anaerobic organisms produce H2S and CS2, which is toxic to most aerobic organisms.
Before the Earth had an oxygen atmosphere, sulfur-reducing bacteria ruled the Earth. When oxygen appeared, aerobic organisms took over because of the energy advantage. Sulfur-reducing bacteria retreated underground where there is no oxygen. On occasion the sulfur bacteria make a comeback, such as during the runaway global warming event 251 million years ago. During this episode, the atmosphere became flooded with H2S, causing a mass extinction of aerobic species.
Peter Ward's book "The Medea Hypothesis" has a nice discussion of the rivalry between aerobic and anaerobic organisms, and of the interaction between the Earth's geology and biology.
In the film "Avatar", the atmosphere of Pandora is toxic to humans because of H2S.
Oxygen is more electronegative than sulfur, which is why reacting hydrocarbons with oxygen yields more energy than by reacting them with sulfur.
The only element that rivals oxygen's electronegativity is fluorine, but fluorine gas is highly reactive and HF is a strong acid.
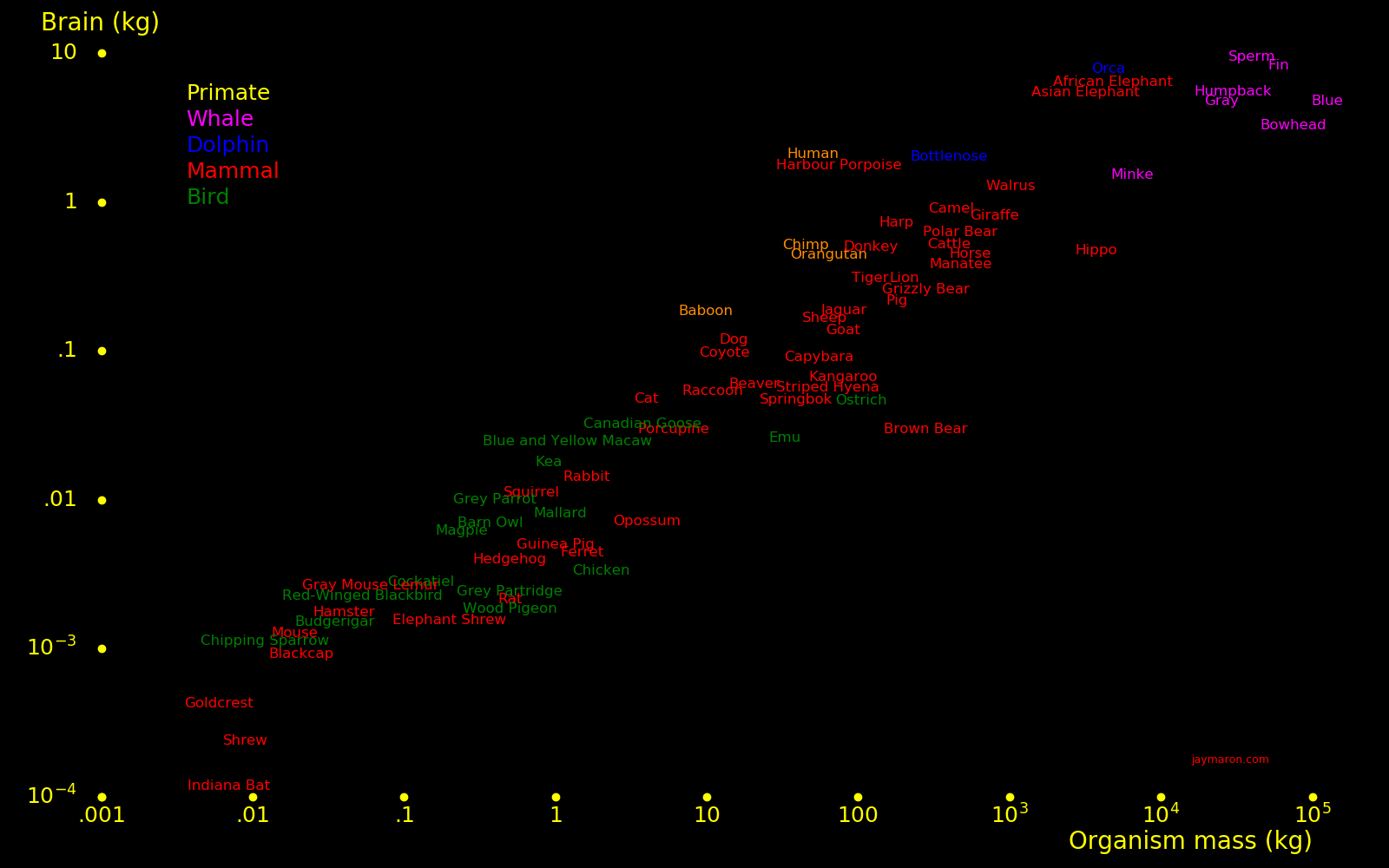 |
|---|
The human brain consumes 20 Watts.
Even though the brain is a power-hungry organ, organisms take the trouble to develop large brains.
Brain Total Brain/
mass mass body
(kg) (kg)
Sperm whale 8
Orca whale 6
Elephant 5 2800 .0018
Blue whale 4
Bottlenose dolphin 1.7
Neanderthal 1.9
Human 1.6 64 .025
Gorilla .6
Chimpanzee .4
Orangutan .4
Horse .4 235 .0017
Pig .20
Dog .10 12 .0080
Cat .04 4 .0091
Squirrel .009 1.4 .0067
Mouse .001 .04 .025
Shrew .0002 .002 .1
Bat .0001
Frog .0058
Small bird .071
Shark .0004
Ant .14
"The body cannot exist without the mind" - Morpheus
 |
|---|
MJoules/kg
Antimatter 90 billion
Hydrogen bomb 25000000 theoretical maximum yield
Hydrogen bomb 21700000 highest achieved yield
Uranium 20000000 as nuclear fuel
Hydrogen 143
Natural gas 53.6
Gasoline 47
Jet fuel 43
Fat 37
Coal 24
Carbohydrates & sugar 17
Protein 16.8
Wood 16
Lithium-air battery 9
TNT 4.6
Gunpowder 3
Lithium battery 1.3
Lithium-ion battery .72
Alkaline battery .59
Compressed air .5 300 atmospheres
Supercapacitor .1
Capacitor .00036
The chemical energy source with the highest energy/mass is hydrogen+oxygen, but
molecular hydrogen is difficult to harness. Hydrocarbons + oxygen is the next
best choice. Carbon offers a convenient and lightweight way to carry hydrogen
around.
Reacting hydrocarbons in an oxygen atmosphere yields the optimal power-to-weight ratio.
Given the enormous power required by brains, if intelligent life exists in the universe, it likely gets its energy from reacting hydrocarbons in an oxygen atmosphere. Most likely we would be able to eat their food.
MJ/kg Calories/gram
Sugar 16 5
Protein 17 5
Alcohol 25 7
Fat 38 9
 |
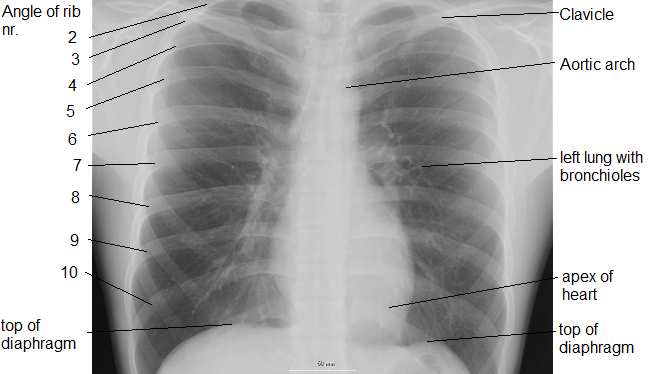 |
|---|---|
Bruce Lee: When the opponent expands I contract, when he contracts I expand, and when there is an opportunity, I do not hit, it hits all by itself.
The diaphragm creates pressure in the abdomen, which expands the ribcage and creates negative pressure for inhalation. Any air-breathing alien has to find a way to generate negative pressure. The ribcage expansion also puts tension energy into the rib muscles, which can be released in a sudden pulse to launch motion of the limbs.
Breathing is coordinated with skeletal motion to minimize energy expenditure. Motion cycles between the following two columns.
Breathe in Breathe out Diaphram contracts Diaphram expands Ribcage expands Ribcage contracts Spine muscles contract Spine muscles release Arms rotate out Arms rotate in Elbows rotate out Elbows rotate in Thumbs rotate out Thumbs rotate in Head rises Head descends Lower back arches Lower back sags Legs rotate out Legs rotate in Gut squashed by diaphram Gut expands Daydream Focus Rebalance Exertion Arms out Arms in High moment of inertia Low moment of inertia Discard angular momentum Discard pressureWhen you are in action, your breathing adjusts to support the timing of your skeletal motion, and if it has any spare time, it sucks in as much air as possible.
When you are relaxing, your breathing adjusts to minimize energy, coordinate cycles, and smooth transitions.
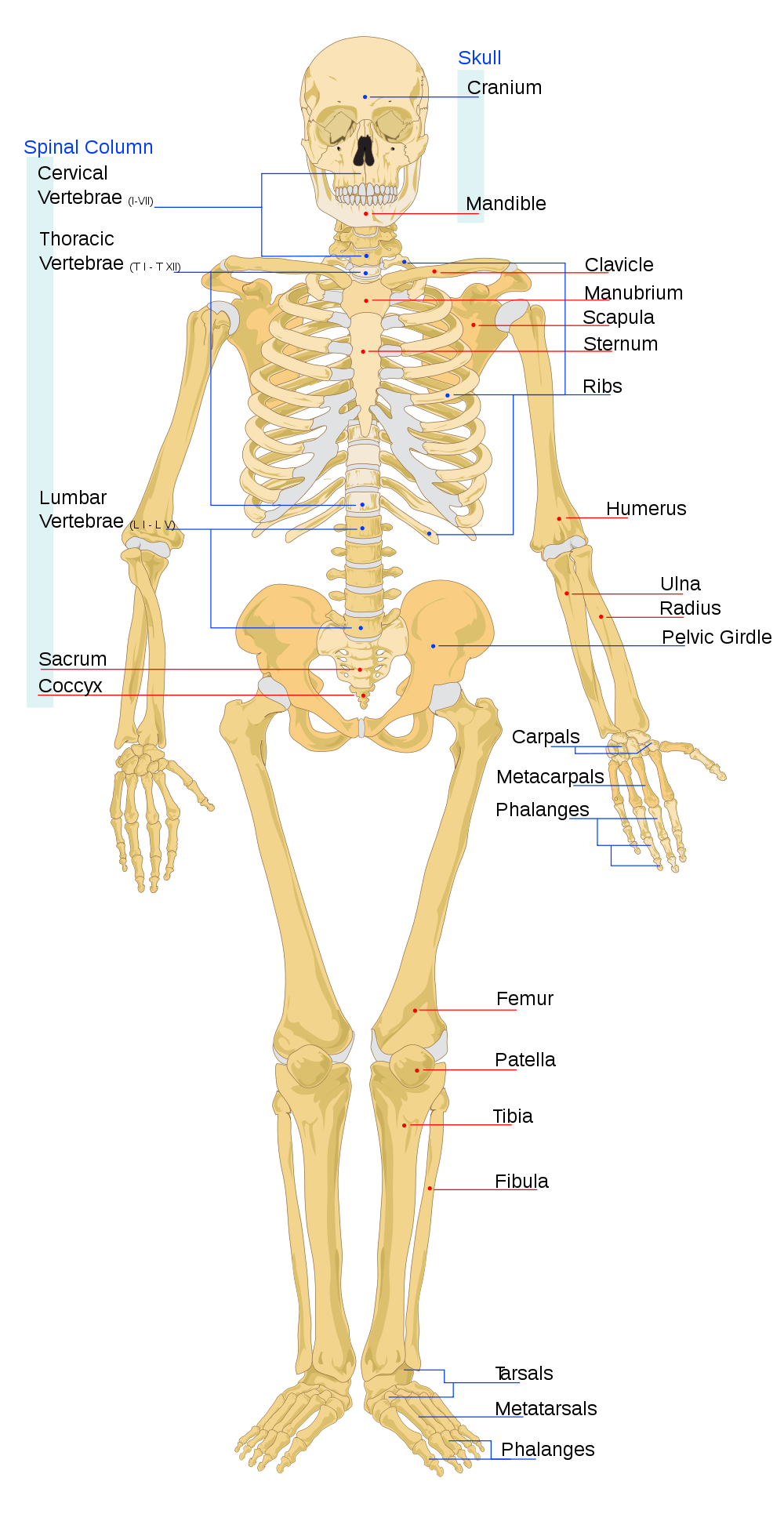
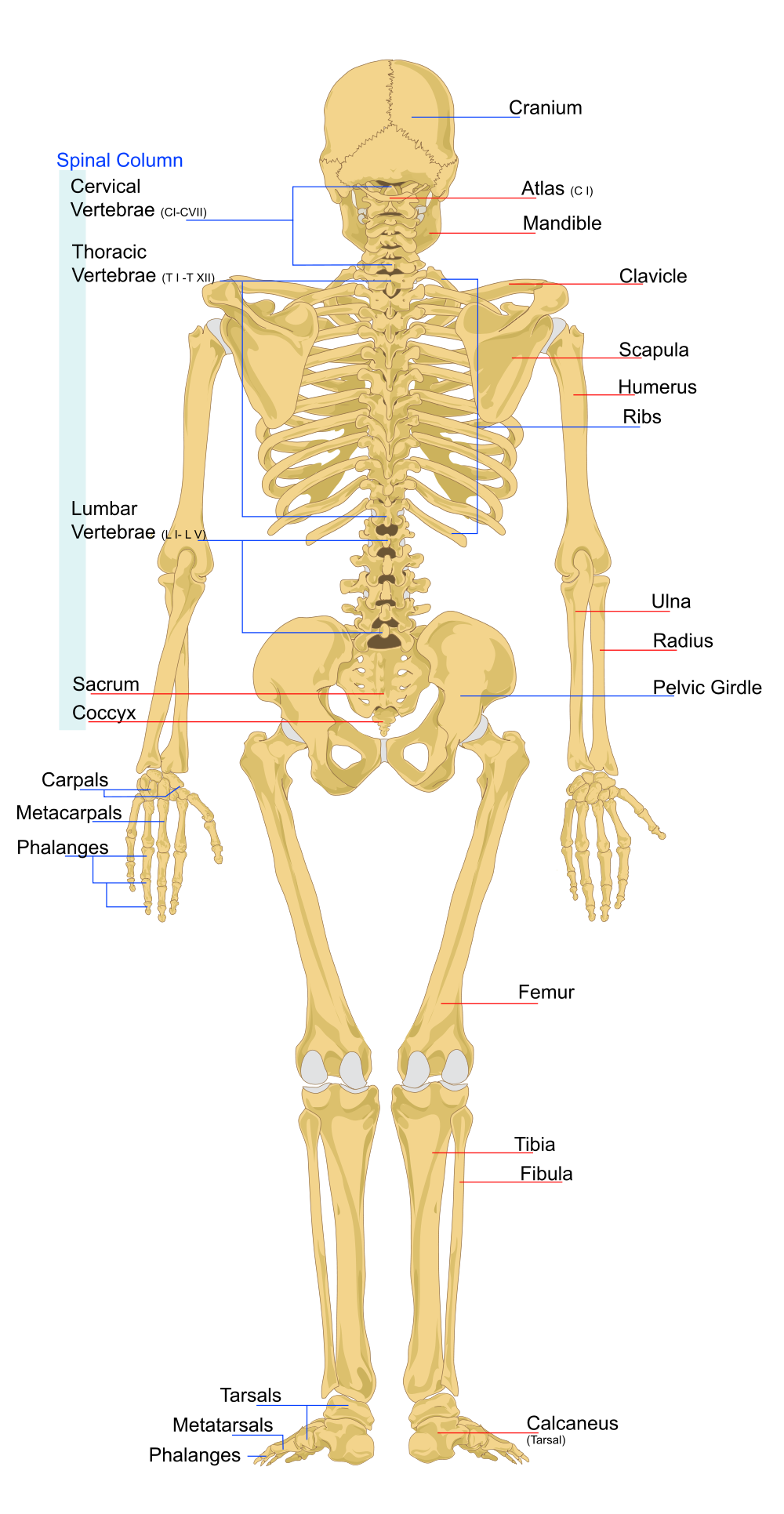
Tetrapod limbs have 2 long bones. A limb with only one long bone is obviously insufficient and 2 long bones are sufficient to support 3D motion of the hand. 3 long bones is probably unnecessary.
There is 1 bone in the upper limb and 2 bones in the lower limb, with a universal joint at the shoulder/hip, a locking joint at the knee/elbow, and a universal joint at the wrist/ankle. The shoulder/hip universal joints can be realistically supported because the torso has abundant muscle mass and torque. The locking joint is harder to support because the upper limb has less muscles and torque than the torso. This is why the lower limb has 2 bones, to help with torque and to stabilize the hand.
The hand is small enough that it can be supported by a univeral joint (the wrist).
The length of limbs is limited by the ability of the torso to deliver force and torque to the hands and feet.
Humans have the most complex wrists in the animal kingdom. Only humans can fully exploit the universal joint of the wrist.
Humans are one of the only animals capable of good balance while on one foot.
 |
 |
 |
 |
|---|---|---|---|
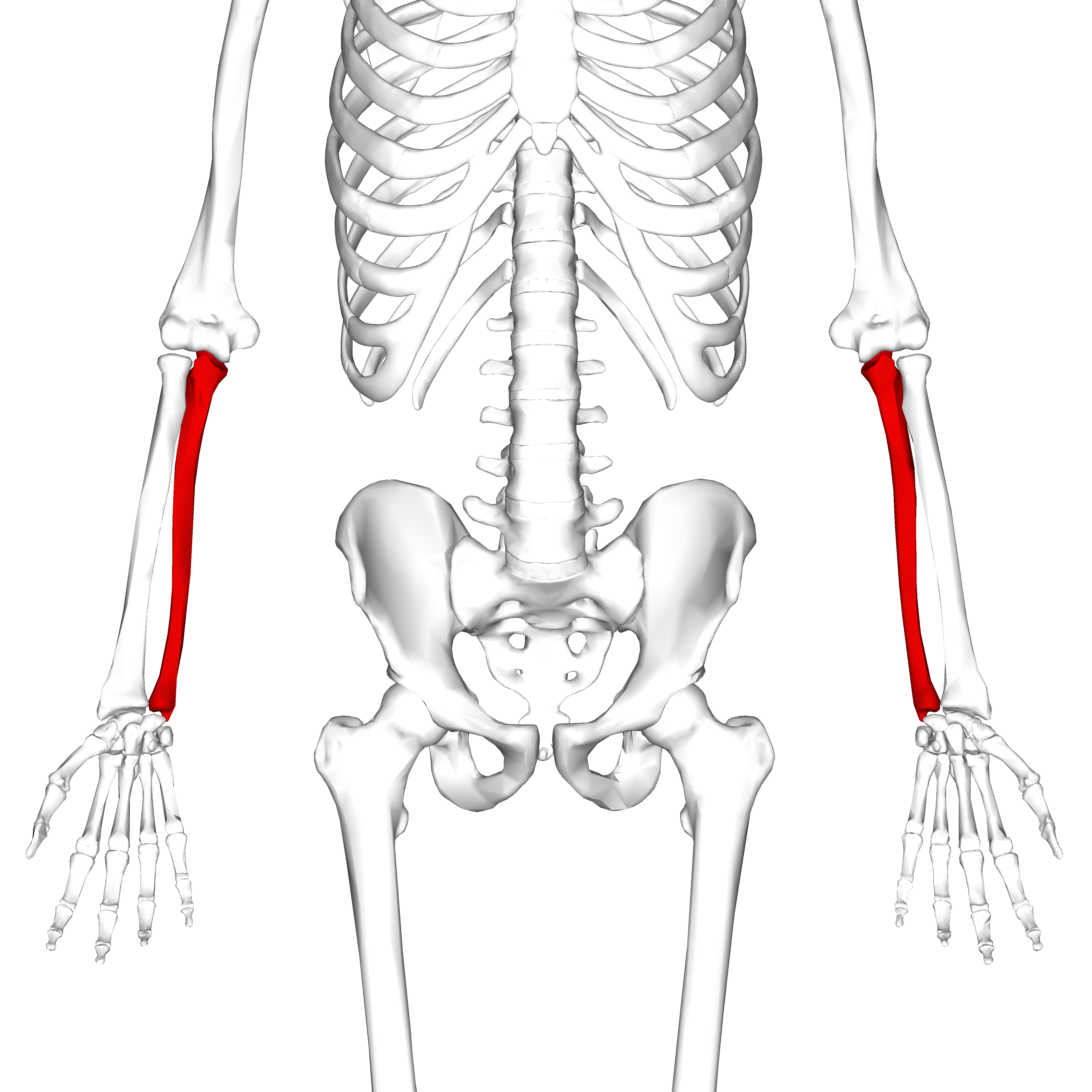
In your forearm, the ulna is the large bone and the radius is the small bone. The forearm should ideally rotate around the large bone.
The ulna connects to your hand on the pinky side and the radius connects to the thumb side. Your hand should rotate about the pivot point where your ulna connects to your wrist.
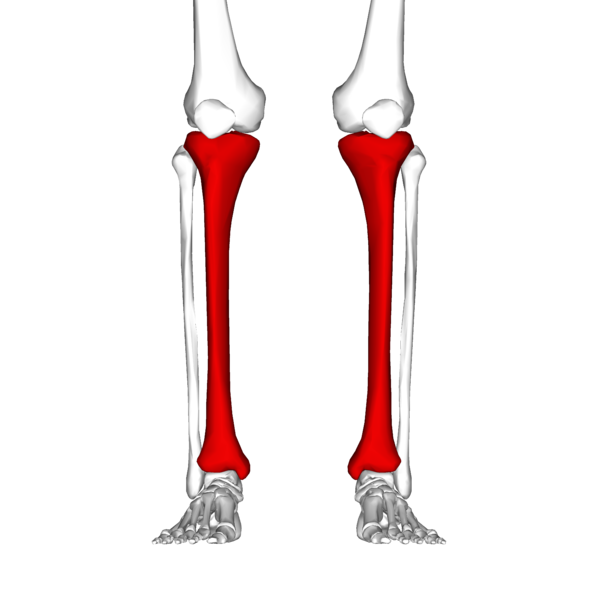
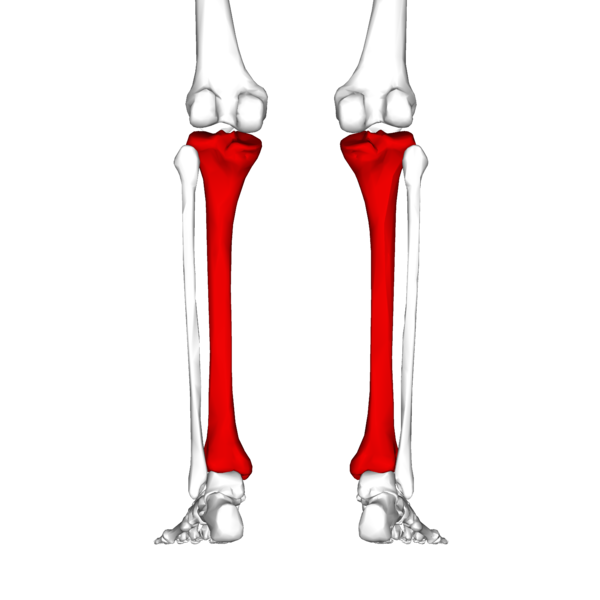
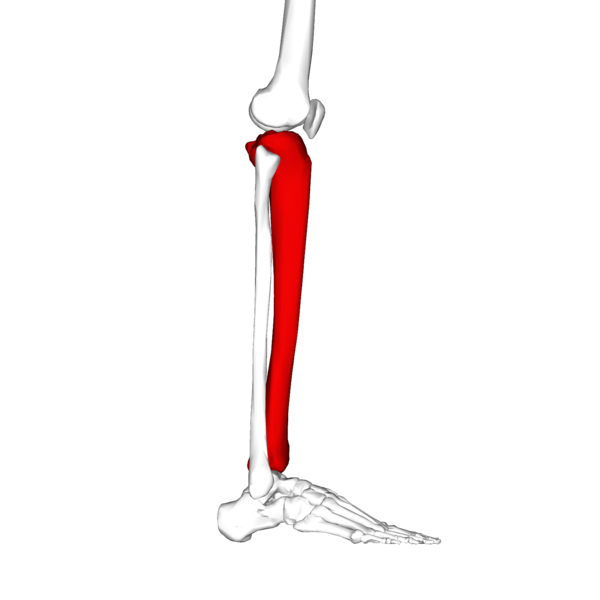
In your lower leg the tibia is the large bone and the fibula is the small bone.
The tibia connects to your foot at the big toe side and the fibula connects at the little toe site.
The lower limbs are connected directly to the spine so that the can deliver force from the ground to the spine. The upper limbs are not directly connected to the spine so that they can absorb shock and move with precision. If an organism has no collarbone then there is no skeletal connection between the limbs and spine at all. If an organism has a colarbone then the connection sequences is:
Spine - Ribs - Breatbone - Collarbone - Shoulder blade - HumerusThe function of the spine is to smooth out angular momentum generated by the limbs.
 |
 |
 |
 |
|---|---|---|---|
Density Pressure Escape Gravity N2 O2 N2 CO2 Ar H2 H3 CH4 Temp
kg/m^3 (Bar) km/s m/s^2 kg/m^3 frac frac frac frac frac frac frac (K)
Venus 67 92.1 10.36 8.87 2.34 .035 .965 735
Titan 5.3 1.46 2.64 1.35 5.22 .984 .014 94
Earth 1.2 1 11.2 9.78 .94 .209 .781 .00039 .0093 287
Mars .020 .0063 .64 5.03 3.8 .00054 .0013 .027 .953 .016 210
Gravity H2 He CH4 Escape
m/s^2 frac frac frac speed (km/s)
Sun 279.4 .735 .248 617.7
Jupiter 24.79 .90 .10 .003 59.5
Saturn 10.44 .96 .03 .004 35.5
Uranus 8.69 .83 .15 .023 21.3
Neptune 11.15 .80 .19 .015 23.5
Titan is the smallest object with an atmosphere and Mercury is the largest object without an atmosphere.
Atmos Atmos Max Min
Density Pressure Gravity N2 O2 N2 CO2 Temp Lapse Height height Height
kg/m^3 (Bar) m/s^2 kg/m^3 frac frac frac (K) (K/km) (km) (km)
Mars .020 .0063 3.71 .00054 .0013 .027 .953 210 4.5 15 22 -7.15
Titan 5.3 1.46 1.35 5.22 .984 94 1.3 14 .5 -2
Venus 67 92.1 8.87 2.34 .035 .965 735 10.5 7 11
Earth 1.28 1.0 9.78 .94 .209 .781 .00039 287 9.8 9 8.8 -0.8
Moon 0 0 1.62 220 8 -6
Ceres 0 0 .27 168
"Atmospheric height" is the height at which the atmosphere density is exp(-1) times the
density at sea level.
"Lapse" is the adiabatic lapse rate.
"Max height" is the maximum elevation.
"Min height" is the minimum elevation.
The atmosphere of Titan is non-toxic and there is enough atmospheric pressure that you don't need a pressure suit. You can roam around Titan with a ski suit and scuba gear. Gravity is so low that human-powered flight is easy.
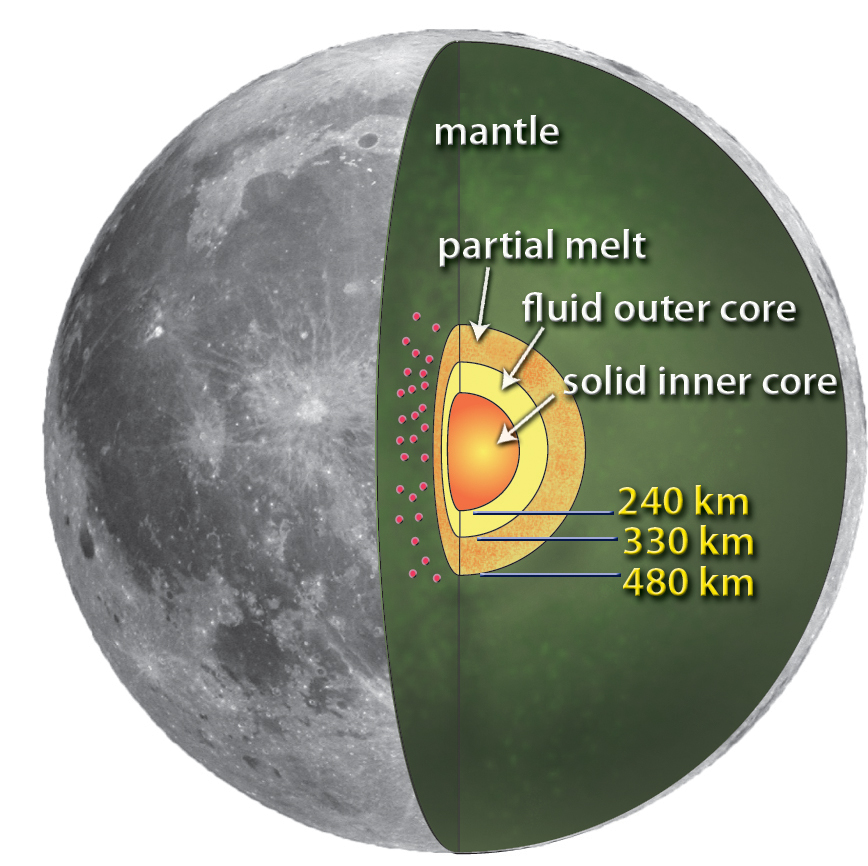 |
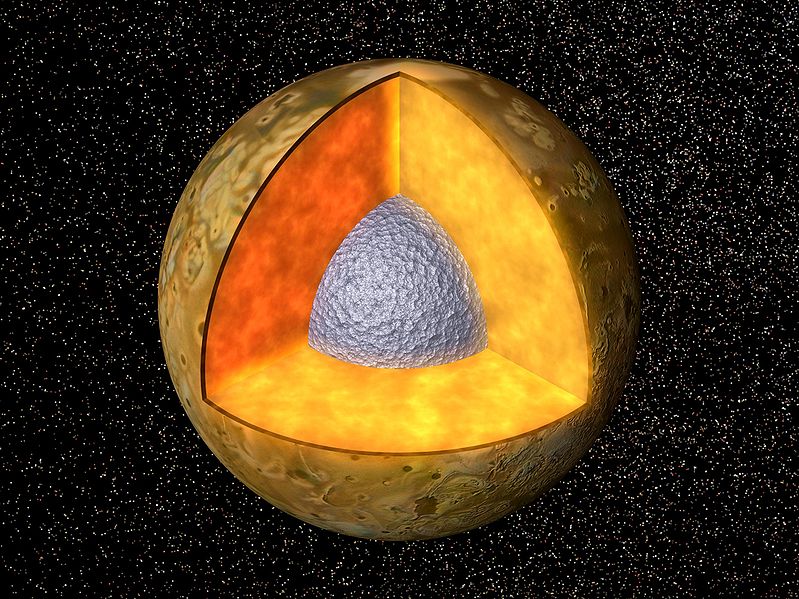 |
|---|---|
Watts/kg half life mantle Watts/kg
of isotope (years) abundance of mantle
uranium-238 9.46e-5 4.47e9 30.8 e-9 2.91 e-12
uranium-235 56.9 e-5 .70e9 .22e-9 .125e-12
thorium-232 2.64e-5 14.0 e9 124 e-9 3.27 e-12
potassium-40 2.92e-5 1.25e9 36.9 e-9 1.08 e-12
The Earth loses heat at a rate of .087 Watts/m^2, for a global heat los
of 4.42e13 Watts.
80% of the Earth's heat is from radioactivity and 20% is from accretion.
The radioactive heating rate 3 billion years ago is twice that of today.
The Earth's core temperature is ~ 7000 K.
Io is heated by tidal forces from Jupiter.
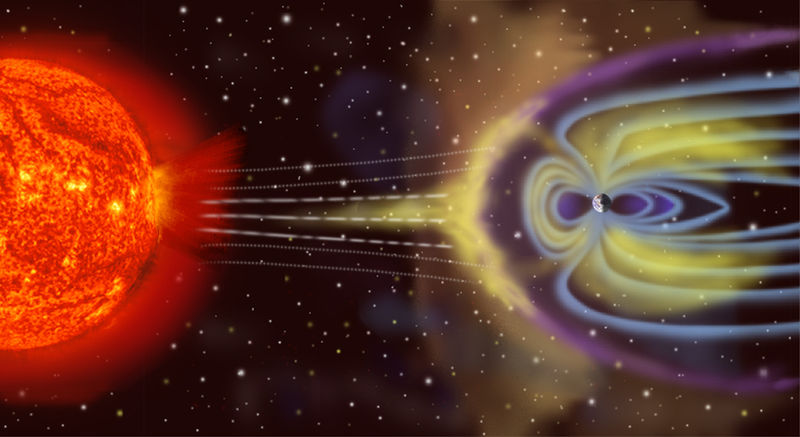 |
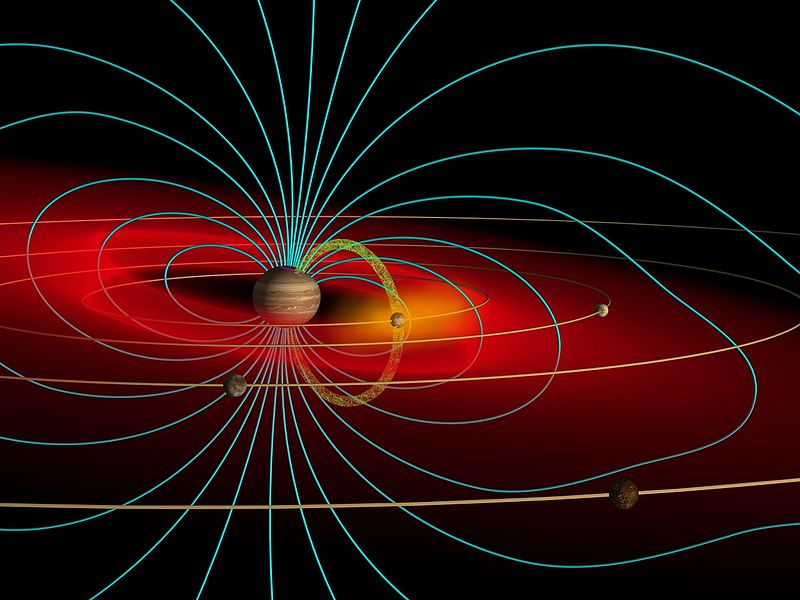 |
|---|---|
Dipole Field at Magnetopause Axis Rotation Volcanic
moment equator (planet angle (days)
(Earth=1) (Gauss) radii) (degrees)
Sun 5 million 25.0
Mercury .0007 .003 1.5 14 58.6 No
Venus <.0004 <.00003 - - 243.0 Yes
Earth 1 .305 10 10.8 1.00 Yes
Mars <.0002 <.0003 - - 1.03 No
Jupiter 20000 4.28 80 9.6 .41
Saturn 600 .22 20 <1 .44
Uranus 50 .23 20 58.6 .72
Neptune 25 .14 25 47 .67
Io Yes
Europa .0016 .0072 4.5
 |
 |
 |
|---|---|---|
 |
|---|
.tif.jpg) |
 |
 |
|---|---|---|
Goldreich & Tremaine (1980): "We present an illustrative application of our results to the interaction between Jupiter and the plantary disk. The angular momentum transfer is shown to be so rapid that substantial changes in both the structure of the disk and the orbit of Jupiter must have taken place on a time scale of a few thousand years."
 |
|---|
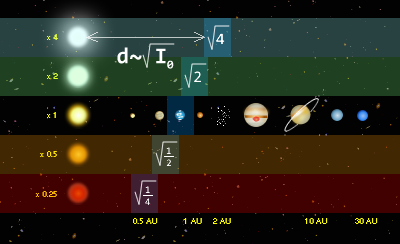
This is a plot of all known planetary systems with at least 3 planets.
Each row corresponds to a planetary system and the solar system is 2/3 of the way down.
Dot size = (Planet Mass)^(1/3) Dot X coordinate = Log(distance from star) Yellow dot = Planets orbit a star with high metallicity Red dot = Plaents orbit a star with low metallicityThe magenta dot indicates the location of the Goldilocks zone. For a given star, Radius of Goldilocks zone ~ (1 AU) * (Star luminosity / Sun luminosity)^(-1/2)
Horizontal cyan lines indicate the range between the planet's perigee and apogee.
Horizontal green lines correspond to 6 times the planet's Hill radius, a measure of the planet's zone of gravitational dominance.
Vertical blue lines indicate the planet's orbital inclination.
 |
|---|
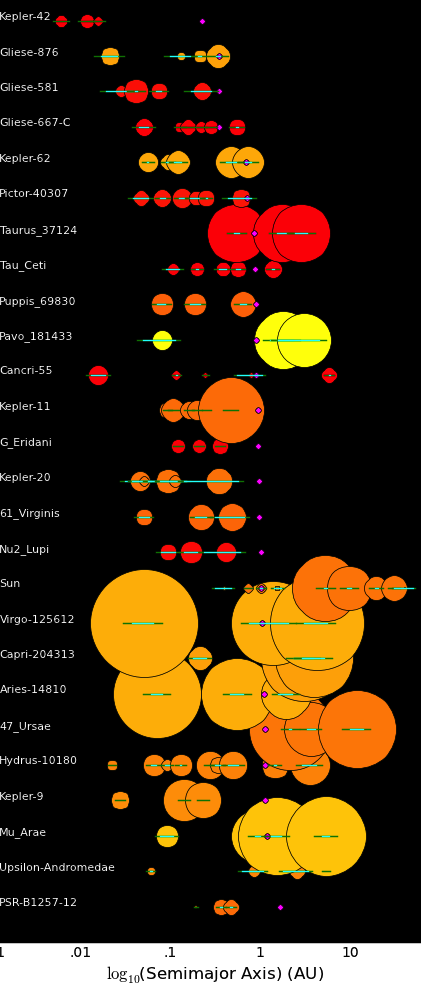
Same as above except for:
Horizontal yellow line = Range from perigee to apogee Horizontal red line = 6 times the planet's Hill radiusThe planets of the solar system are smaller and more widely spaced than what is typical for exoplanetary systems. In the Galactic Museum of Natural History, the solar system might be classified as a "Dwarf planetary system".
 |
 |
 |
|---|---|---|
Planet property If too little If too much
Mass Cannot capture atmosphere Becomes gas giant
No volcanism
Cannot generate a magnetic field
Distance from Too hot Too cold for surface water
star Inside the snow line
Atmospheric Cosmic rays reach the surface Blocks too much sunlight
thickness Atmosphere loses heat at night for photosynthesis
Water content If you don't have oceans then you No dry land
don't have enough photosynthesis
to generate an oxygen atmosphere
Planet spin Does not generate a large-scale
magnetic field
Planet spin tilt Extreme seasons
Star temperature Not enough blue light for Too much UV light
photosynthesis
Star metallicity Small planets Too many gas giants
Star mass Planet is so close to the star that it
is tidally locked to the star
Moon mass Planet tilt becomes unstable, causing
extreme seasons
A moon of a gas giant can potentially be protected from the solar wind by the
gas giant's magnetic field. It can also potentially have volcanism from tidal
heating by the gas giant.
The Earth has been beset by asteroids, supervolcanoes, global ice ages, runaway global warming, supernovae, gamma ray bursts, and the industrial age.
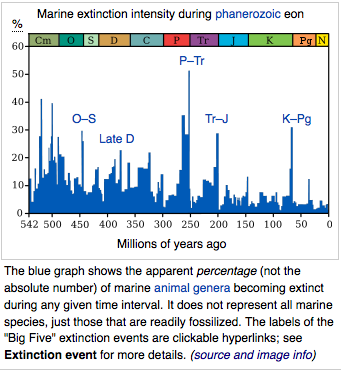 |
|---|
Millions of
years ago
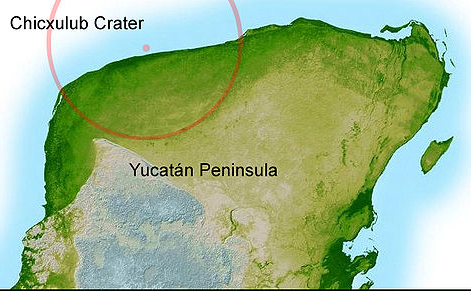
66 Cretaceous–Paleogene extinction, caused by a 10 km asteroid.
Dinosaurs become extinct.
201 Triassic-Jurassic extinction. Cause unknown.
252 Permian-Triassic extinction. Runaway global warming
370 Late-Devonian extinction. Cause unknown.
445 Ordovician-Silurian extinction events. Global glaciation.
 |
 |
|---|---|
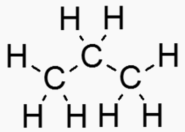
Molecules are often depicted with the hydrogens excluded.
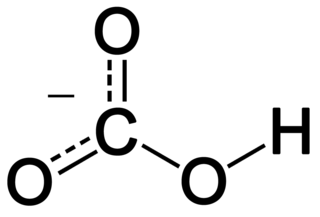
Functional Organic group molecule C-H3 Alkane (lipid) C-H2OH Alcohol C-OOH Fatty acid (carboxylic acid) C=O Carbonyl group. Aldehyde if at end, ketone if not at endHumans can metabolize just about any chain hydrocarbon and any sugar.
 |
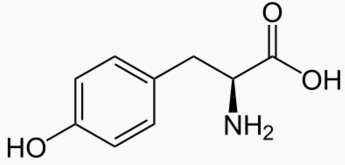 |
|---|---|
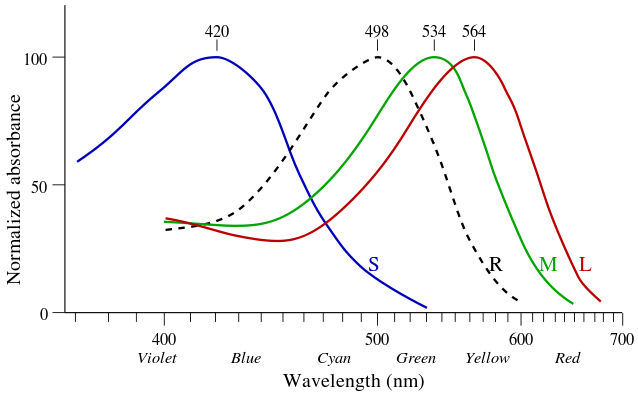 |
|---|
Opsin Wavelength Humans Notes
(nm)
Parapinopsin UV 365 Catfish
Neuropsin 380 Bird vision. Found in the brains of humans
OPN1SW 440 Blue All mammals
Panopsin Blue 450 Fish vision. Found in the brains of humans
Parapinopsin Blue 470 Catfish and lamphrey
SWS2 480 Extinct in mammals
Melanopsin 480 Found in the brains of humans
VA 500 Vertebrates except mammals. Vertebrae ancient opsin.
RH1 500 White Black/White
Panopsin Cyan 500 Fish vision. Found in the brains of humans
Pareitopsin 522 Lizards
OPN1MW 534 Green All mammals
OPN1LW 564 Red Once possessed by mammals, then lost by most
RH2 600 Black/White. Extinct in mammals
Retinal G Found in the brains of humans
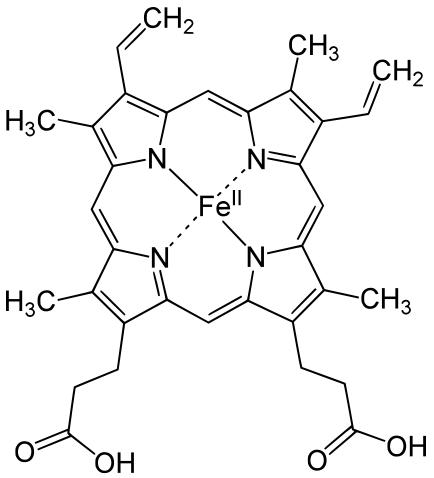 |
 |
|---|---|
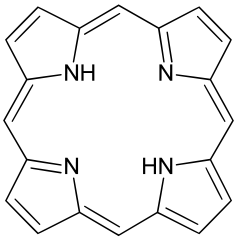
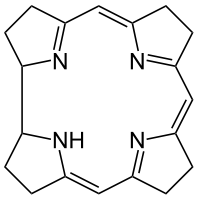
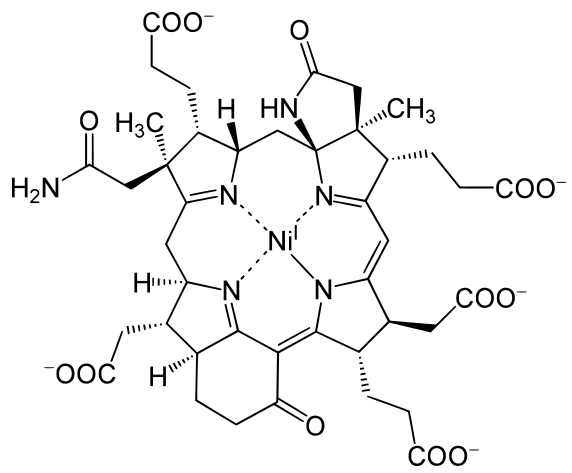
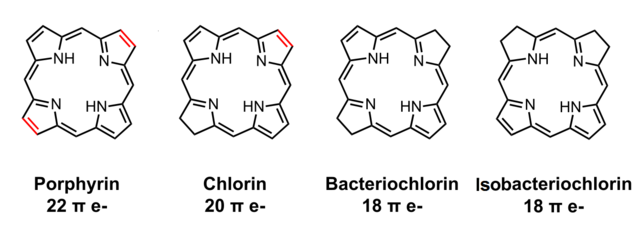
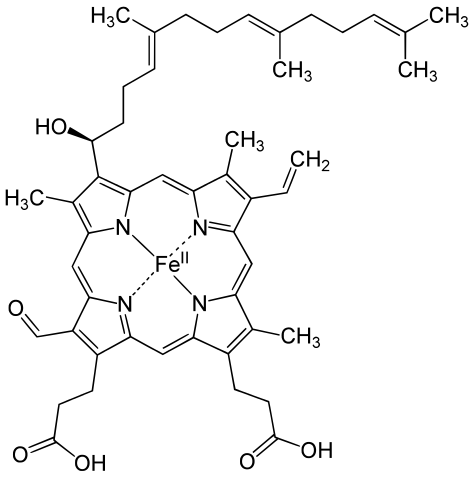
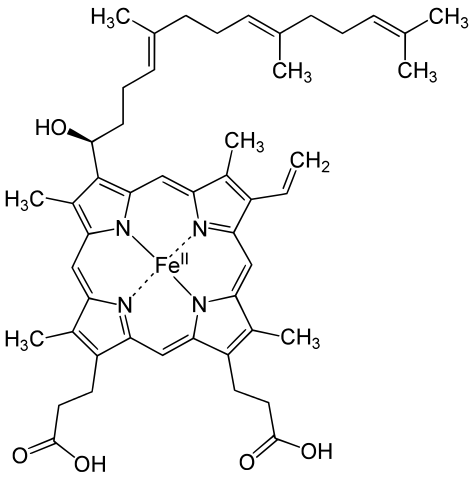
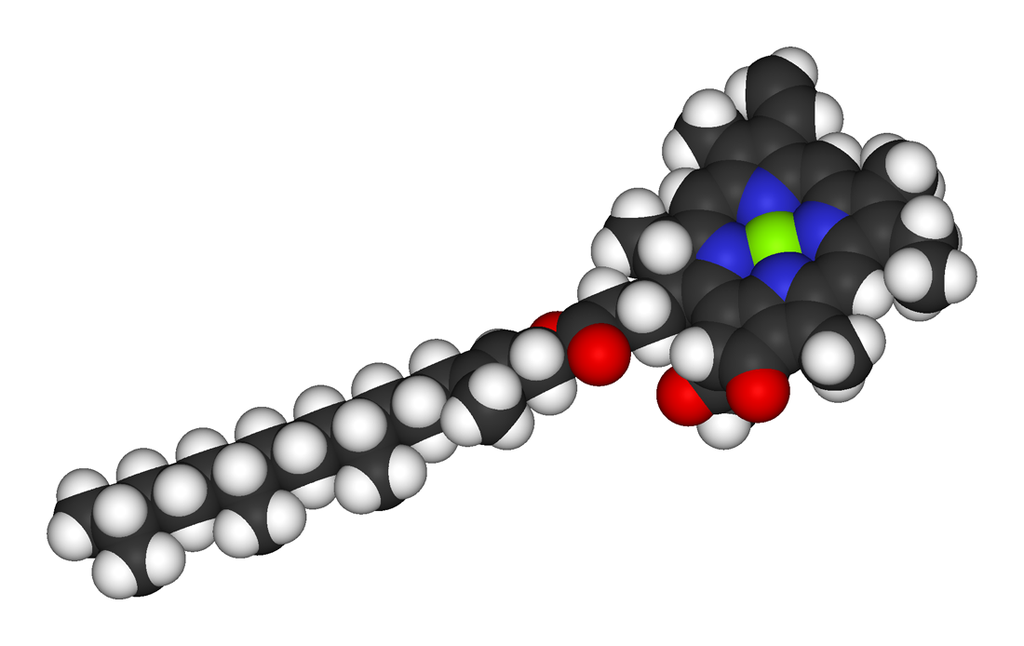
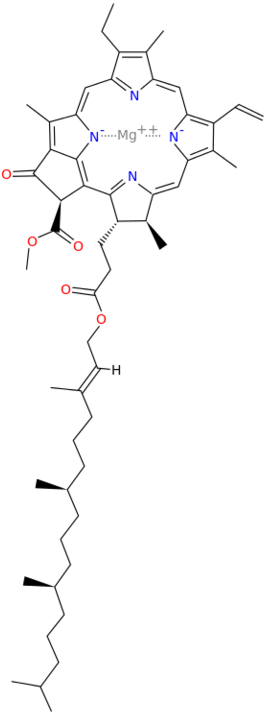
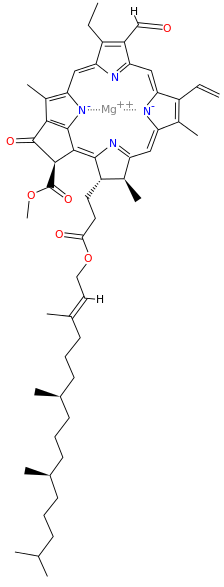
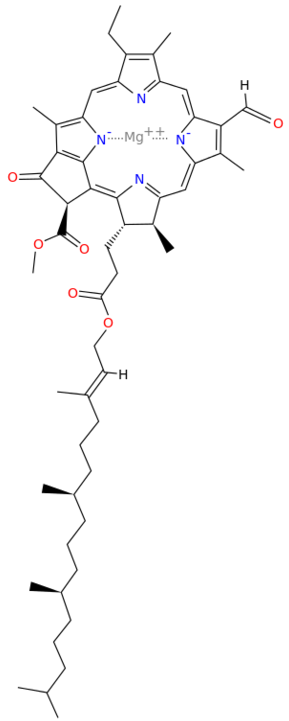
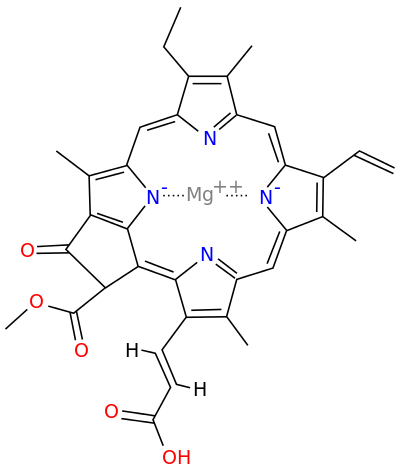
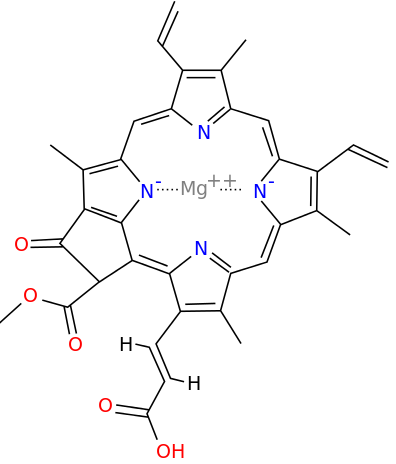
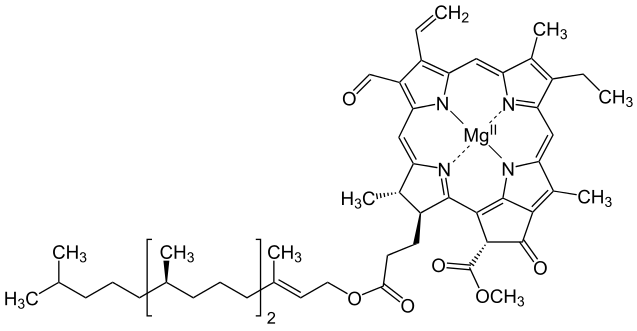
Metals are held by a cofactor, which is held by a protein. Many cofactors are porphyrin rings conposed of 4 pyrroles. Examples of porphyrins:
 |
 |
 |
 |
|---|---|---|---|
 |
 |
 |
 |
|---|---|---|---|
 |
 |
 |
|---|---|---|
 |
|---|
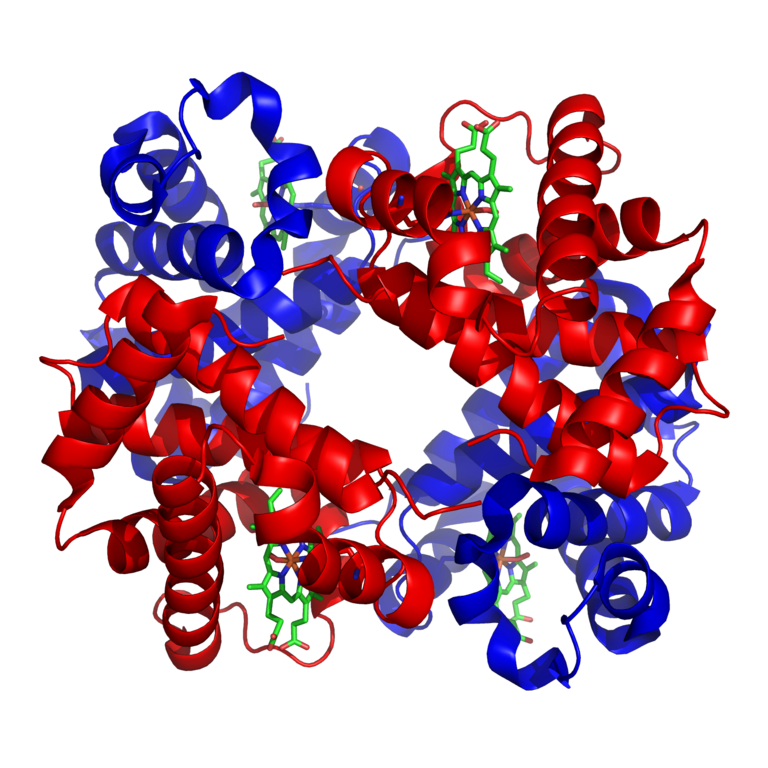
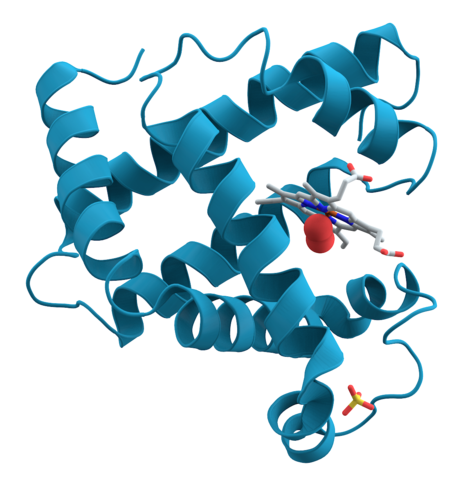
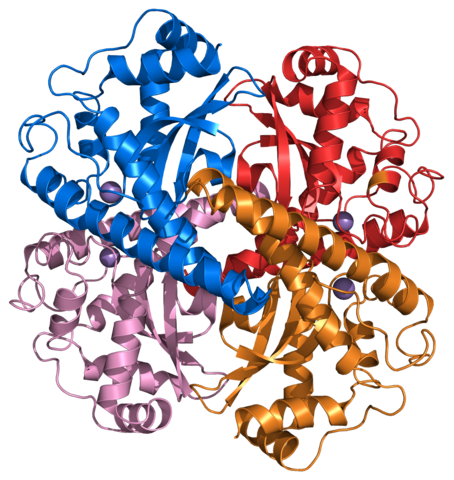
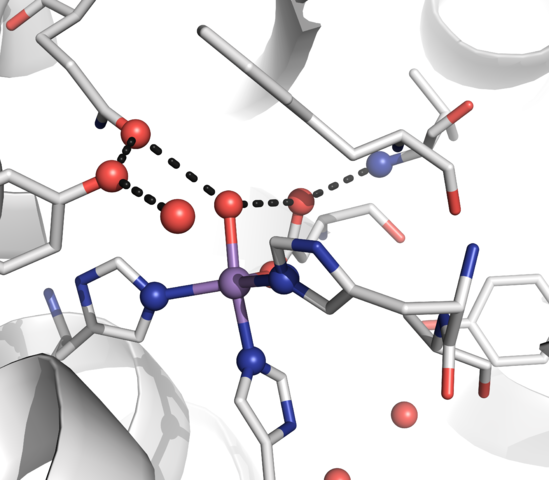
Oxygen bonds to the iron in a heme molecule and becomes superoxide.
Hemoglobin is a set of 4 helix proteins that carry 4 iron ligands, and each
iron ligand carries 1 oxygen molecule.
Human hemoglobin is composed mostly of heme B.
The oxygen density of hemoglobin is 70 times the solubility of oxygen
in water.
Hemoglobin fraction of red blood cells = .96 (dry weight) Hemoglobin fraction of red blood cells = .35 (including water) Oxygen capacity of hemoglobin = 1.34 Liters of oxygen / kg hemoglobin Iron ligands per hemoglobin = 4 O2 molecules per ion ligand = 1
 |
 |
 |
|---|---|---|
 |
 |
 |
|---|---|---|
 |
 |
 |
|---|---|---|
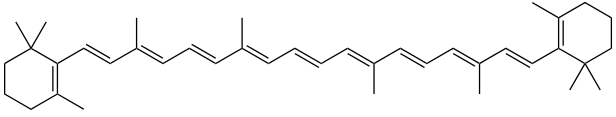
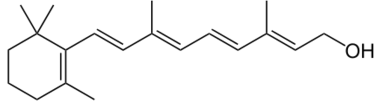
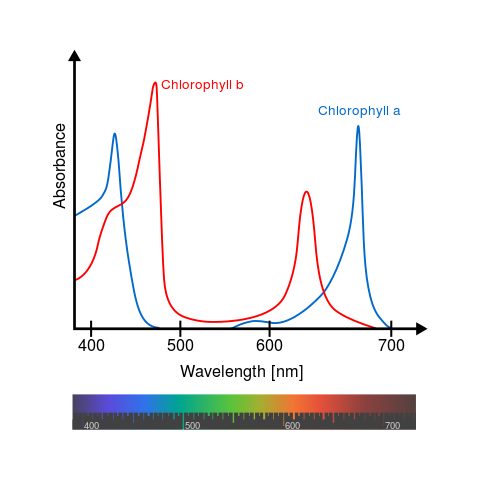
All chlorophyll uses magnesium.
A Universal B Plants C1 Algae C2 Algae D Cyanobacteria F Cyanobacteria
 |
 |
 |
|---|---|---|
 |
 |
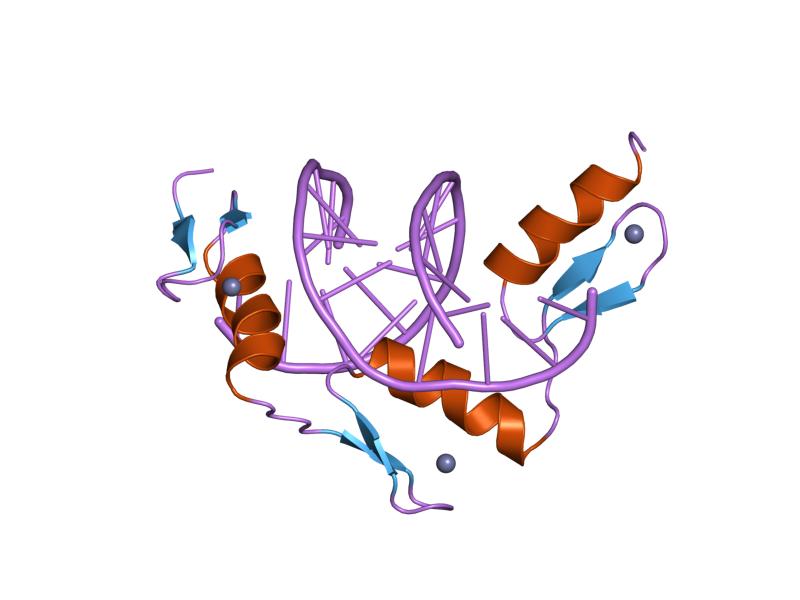 |
|---|---|---|
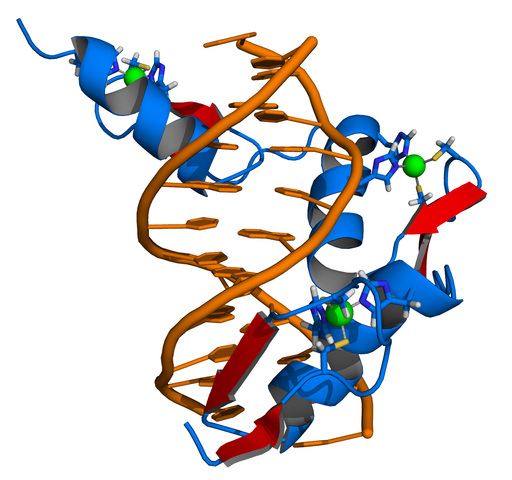
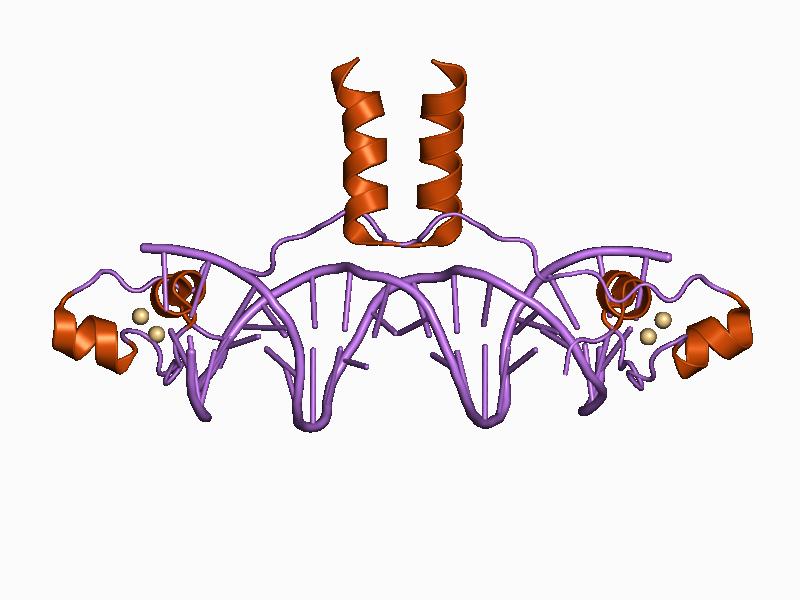
Zinc stabilizes the proteins that manipulate DNA and RNA.
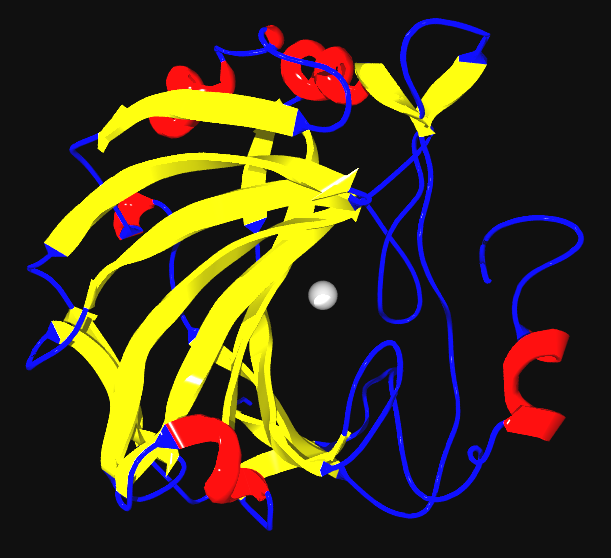 |
|
|---|---|
Element Humans Cofactor Function
Hydrogen *
Helium No biological role
Lithium No biological role
Beryllium Toxic becauseit displaces magnesium in proteins
Boron * Plant cell walls. Metabolism of calcium in plants & animals
Magnesium * Chlorin Chlorophyll
Scandium No biological role
Titanium No biological role
Vanadium Found only in rare bacteria.
Chromium No biological role
Manganese * Superoxide dimutase. Converts superoxide to oxygen
Iron * Porphin Hemoglobin
Cobalt * Corrin Cobalamin (Vitamin B12)
Nickel Corphin Coenzyme F430 (Creates methane. Found only in archaea)
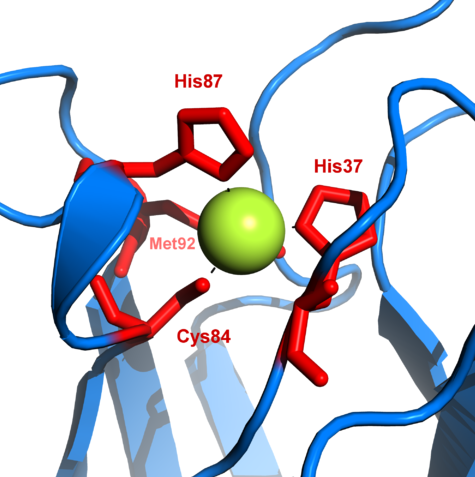
Copper * Heme Cytochrome C oxidase. Electron transport chain
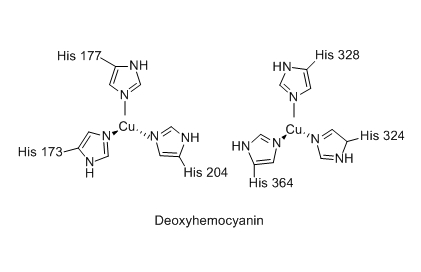
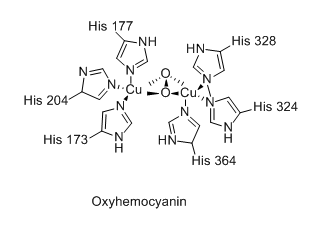
Hemocyanin, an alternative to hemoglobin used by some animals
Hemoglobin carries 4 times as much oxygen as hemocyanin
Plastocyanin protein, used in photosynthesis
Sometimes used in superoxide dimutase
Zinc * Component of proteins that manipulate DNA and RNA (Zinc fingers)
Component of carbonic anhydrase, which interconverts CO2 and HCO3
Metallothionein proteins, which bind to metals such as
zinc, copper, selenium cadmium, mercury, silver, and arsenic
Molybdenum Nitrogen fixase. Convert N2 to NH3
Selenium * Component of the amino acide selenocysteine
Bromine * Limited role
Iodine * Component of thyroxine and triiodotyronine, which
regulate metabolic rate
Lead Toxic because it displaces calcium in bones
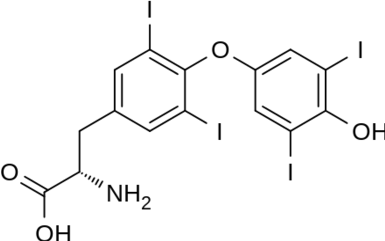 |
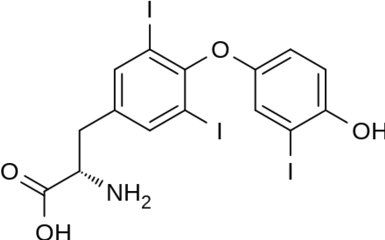 |
|---|---|
 |
 |
 |
|---|---|---|
 |
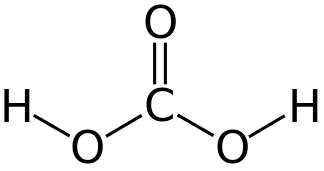 |
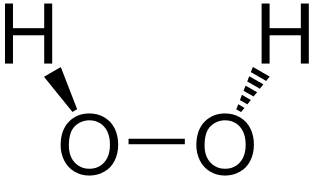 |
|---|---|---|
Superoxide dimutase converts superoxide to oxygen or hydrogen peroxide.
The peroxidase enzyme decomposes hydrogen peroxide to water. Peroxidase contains the selenocysteine amino acid, which contains selenium.
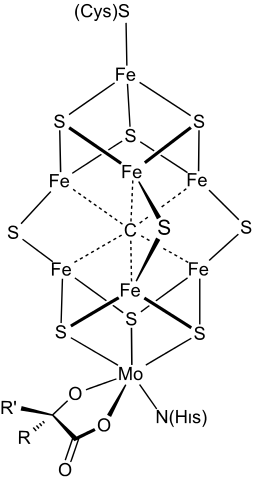 |
|---|
Nitrogen fixase uses an iron-molybdenum cofactor.
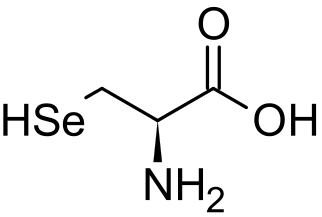 |
|---|
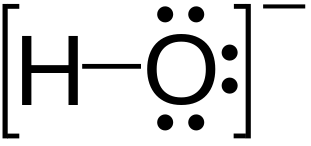
Selenium is a component of the amino acid selenocysteine.
 |
 |
|
|---|---|---|
The hemocyanin protein uses copper to carry oxygen. It has an oxygen density that is 1/4 of hemoglobin.
Plastocyanin is a copper-containing protein used in photosynthesis.
 |
|---|
 |
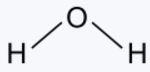 |
|---|---|
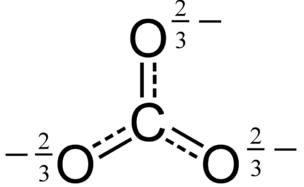 |
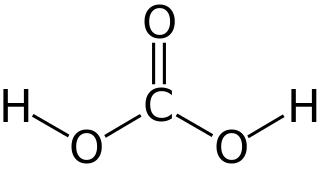 |
|---|---|
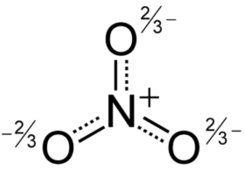 |
 |
|---|---|
 |
 |
|---|---|
 |
 |
|---|---|
 |
 |
|---|---|
 |
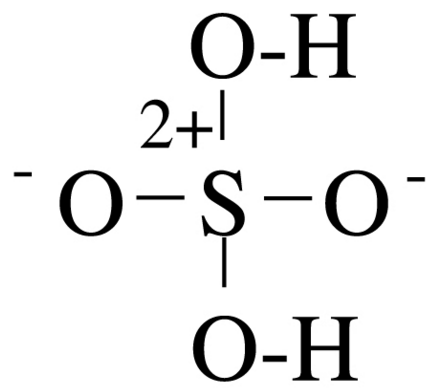 |
|---|---|
 |
 |
 |
|---|---|---|
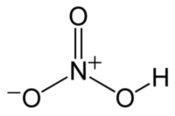
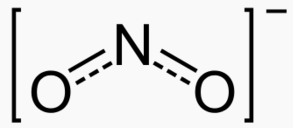
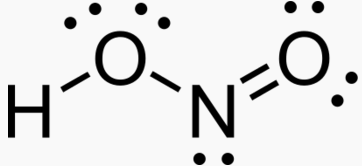
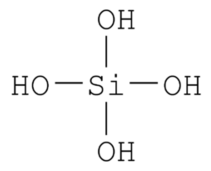
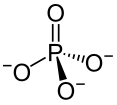
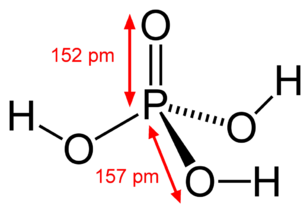
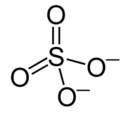
OH- CO3-- NO3- NO2- SiO4---- PO4--- SO4-- Iron(III) Oxide Fe2O3 Ferric oxide. Most common form Iron(II) Oxide FeO Rare Iron(II,III) Oxide Fe3O4 Magnetite Copper(I) Oxide Cu2O Cuprous oxide Copper(II) Oxide CuO Cupric oxide Copper(III) Oxide Cu2O3
 |
|---|
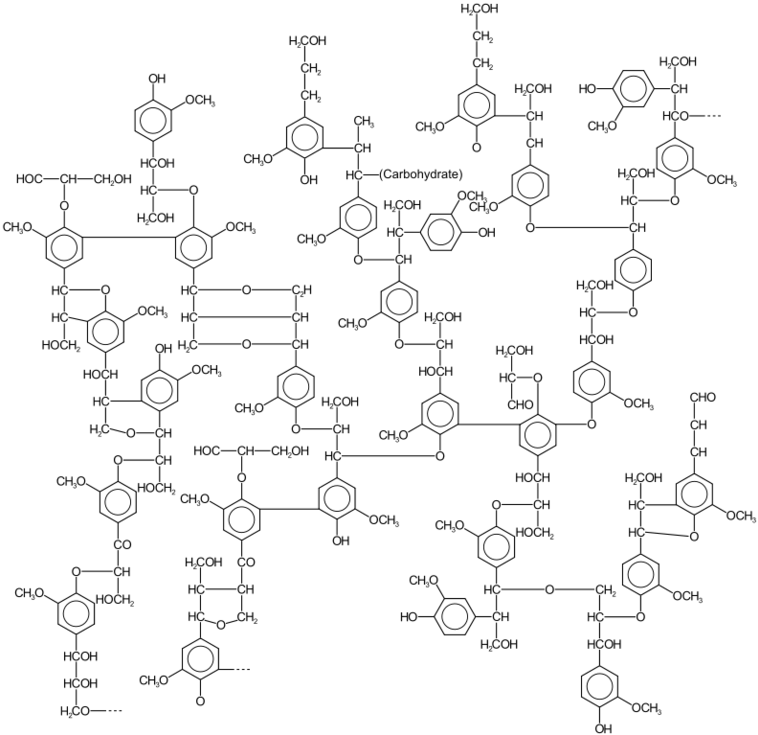
Lignin is the structural component of wood.
 |
 |
 |
|---|---|---|
Audax: Lives underground, eats rock, and gets its energy from molecules generated by radioactivity. It can colonize a planet from scratch.
Conan: Survives radioactivity, outer space, acid, and freezing. It has four independent sets of chromosomes with active repair mechanisms.
Tardigrade (water bear): Survives temperatures from absolute zero to 161 C. Survives outer space. Found everywhere on the Earth from Mount Everest to the bottom of the ocean.
 |
 |
|---|---|
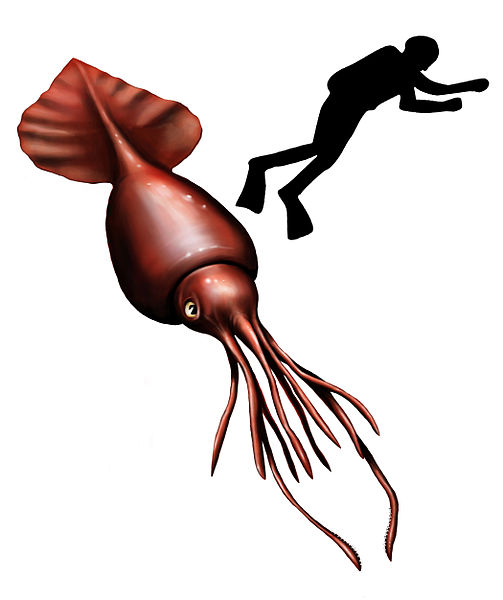
The colossal squid is up to 14 meters long, has eyes up to 27 cm in diameter, and inhabits the ocean at depths of up to 2 km.
 |
 |
|---|---|
The eyes of a Mantis shrimp have 12 color channels, including UV, and they are sensitive to linear and circular polarization. Each eye is trinocular, giving it a total of 6 channels for depth perception.
The Mantis shrimp has two clubs for striking.
Impact speed = 23 m/s Acceleration = 10400 g (similar to a .22 calibre bullet) Impact force = 1500 NewtonsThe strike produces cavitation bubbles that add to the damage.
 |
|---|
Top speed of 33 meters/second
Accelerates from 0 to 28 meters/second in 3 seconds
 |
|---|
Fastest bird. Top horizontal speed of 45 meters/second.
 |
|---|
Mass of up to 15 kg
Wingspan of up to 3.1 meters
Mass of 75 kg
Wingspan of 7 meters
Wing loading of 85 Newtons/meter^2
Wing area of 8.1 meters^2
Extinct
Edward Lasker: While the Baroque rules of Chess could only have been created by humans, the rules of Go are so elegant, organic, and rigorously logical that if intelligent life forms exist elsewhere in the universe, they almost certainly play Go.
 |
 |
|---|---|
 |
|---|
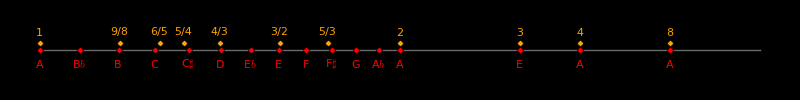
Just tuning is based on integer ratios and equal tuning is based on logarithms, and there is no direct connection between them. Fortuitously, 12-tone equal tuning gives a set of notes that are nearly identical to those for just tuning. The correspondence is close, but not exact, and violinists use a compromise between just and equal tuning that is situation dependent.

The synthesis of just and equal tuning offers rich contrapuntal possibilities, as was explored during the Baroque age by composers such as Vivaldi, Bach, and Handel.
Just and equal tuning
Note Index Interval Equal Just tuning Major Minor Pythagorean
tuning scale scale tuning
A 0 Unison 1.000 1.000 = 1/1 * * 1/1
Bflat 1 Minor second 1.059 256/243
B 2 Major second 1.122 1.125 = 9/8 * * 9/8
C 3 Minor third 1.189 1.200 = 6/5 * 32/27
C# 4 Major third 1.260 1.250 = 5/4 * 81/64
D 5 Fourth 1.335 1.333 = 4/3 * * 4/3
Eflat 6 Tritone 1.414 729/512
E 7 Fifth 1.498 1.500 = 3/2 * * 3/2
F 8 Minor sixth 1.587 * 128/81
F# 9 Sixth 1.682 1.667 = 5/3 * 27/16
G 10 Minor seventh 1.782 * 16/9
Aflat 11 Major seventh 1.888 * 243/128
A 12 Octave 2.000 2.000 = 2/1 * * 2/1
In equal tuning, the frequency ratio of an interval is
exp(Index/12)
Equal tuning is based on equal frequency ratios. Just tuning adjusts the frequencies to correspond to the nearest convenient integer ratio. For example, in equal tuning, the frequency ratio of a fifth is 1.498. Just tuning changes it to 1.500 = 3/2.
The 12-tone scale is ubiquitous in Earth music and it arises from elegant mathematics. If alien life plays music, they likely use the 12-tone scale.
The major and minor scales select 8 notes from the 12 note scale, favoring notes that have nice integer ratios.
In the 6th century BCE, Pythagoras developed a 12-tone scale based on the ratios 2/1 and 3/2. This tuning was widely used until the 16th century CE. Pythagoriean tuning gives good results for fourths and fifths but poor results for thirds.
1572 Bombelli publishes complex numbers
1523 Pietro Anon introduced "meantone tuning" to fix the thirds, using a
frequency ratio of 5/4 for major thirds. His treatise "Thoscanello de la
musica" expanded the possibilities for chords and harmony.
1555 Andrea Amati develops the four-string violin.
1584 "Equal tuning" introduced. Equal tuning divides the octave logarithmically.
The first known examples of equal tuning were:
Vincenzo Galilei in 1584 (Father of Galileo Galilei)
Zhu Zaiyu in 1584
Simon Stevens in 1585
1585 Simon Stevin introduces decimal numbers (For example, writing 1/8 as 0.125).
This greatly expanded the calculational power of numbers.
1586 Simon Stevin drops objects of varying mass from a church tower to demonstrate that
they accelerate uniformly.
1604 Galileo publishes a mathematical description of acceleration.
1614 Logarithms invented by John Napier, making possible precise calculations
of equal tuning ratios. Stevin's calculations were mathematically sound but
the frequencies couldn't be calculated with precision until logarithms were
developed.
1637 Cartesian geometry developed by Fermat and Descartes
1684 Leibniz publishes The Calculus
1687 Newton publishes the "Principia Mathematica"
1722 Bach publishes the "Well Tempered Clavier"
Until ~ 1650, most keyboards used meantone tuning. This tuning gives good results if you
confine yourself to a small number of keys and use few accidentals but it can't be made
to work for all keys.
J.S. Bach tuned his own harpsichords and clavichords and he customized the tuning to
work in all 24 keys ("well temperament"). He demonstrated its effectiveness
in "The Well Tempered Clavier".
1821 Cauchy publishes the "Cours d'Analyse", introducing rigor to mathematics.
 |
 |
 |
|
|---|---|---|---|
In the baroque age, violinists played with a pure, vibratoless tune, using bow speed rather than vibrato for expressivity. After the baroque age, an epidemic of vibrato emerged and is still with us, especially at Juilliard and Lincoln Center. Vibrato obstructs the resonances of just intonation.
"There are performers who tremble consistently on each note as if they had the permanent fever" - Leopold Mozart, 1756
The violinist and teacher Leopold Auer, in his book "Violin Playing as I Teach It" (1920), advised violinists to practise playing completely without vibrato, and to stop playing for a few minutes as soon as they noticed themselves playing with vibrato in order for them to gain complete control over their technique.
From the Wikipedia page on Yehudi Menuhin: After building early success, he experienced considerable physical and artistic difficulties caused by overwork during the war as well as unfocused and unstructured early training (reportedly he said "I watched myself on film and realized that for 30 years I'd been holding the bow wrong"). Careful practice and study combined with meditation and yoga helped him overcome many of these problems. When he finally resumed recording, he was known for practising by analyzing music phrases one note at a time.
 |
 |
 |
|---|
 |
 |
 |
|---|---|---|
 |
 |
 |
|---|---|---|
 |
 |
|---|---|
 |
 |
 |
|---|---|---|
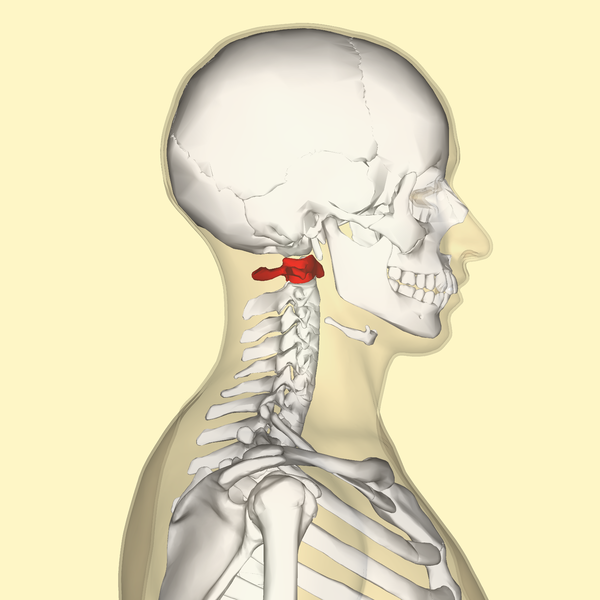
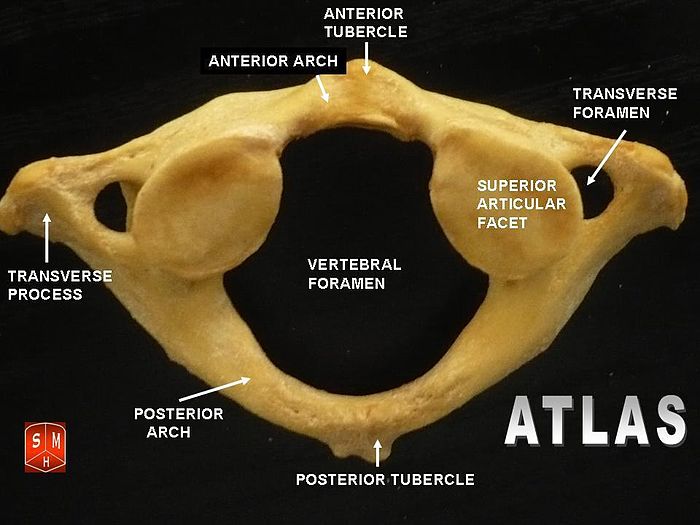
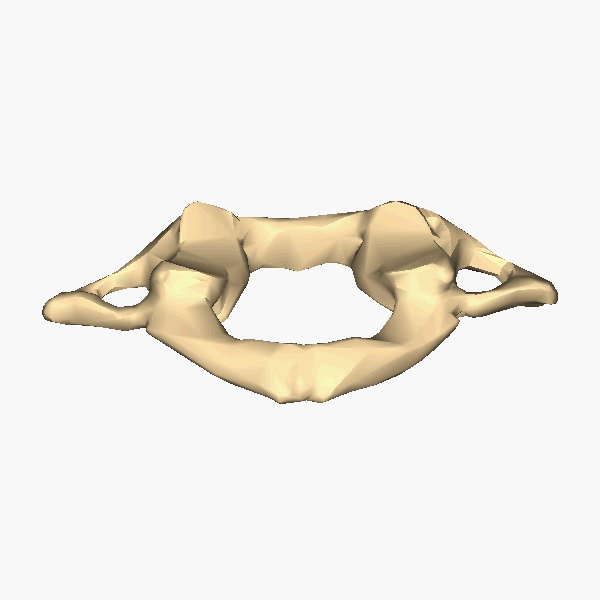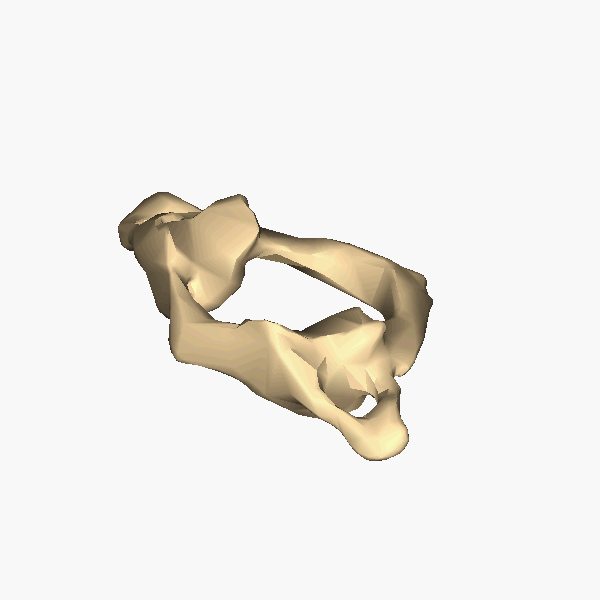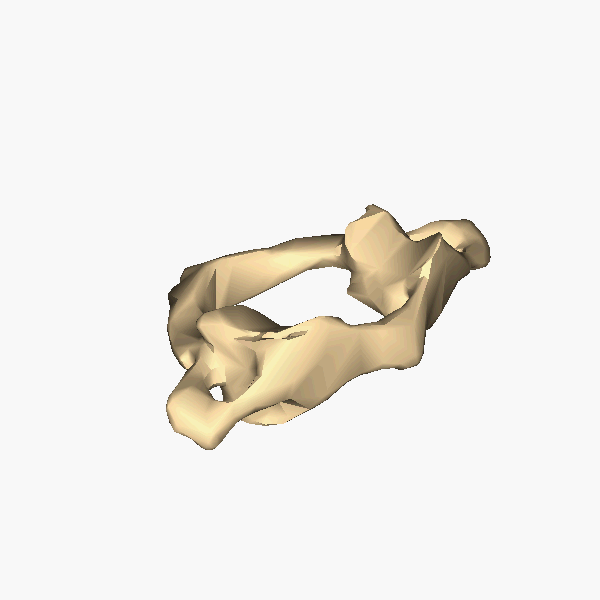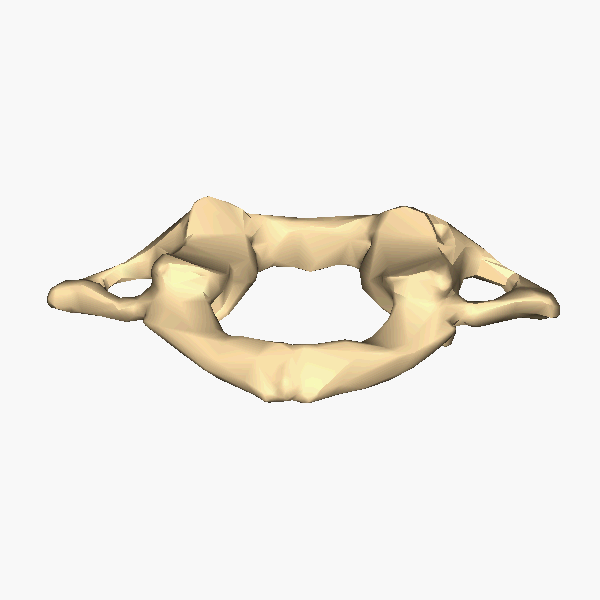
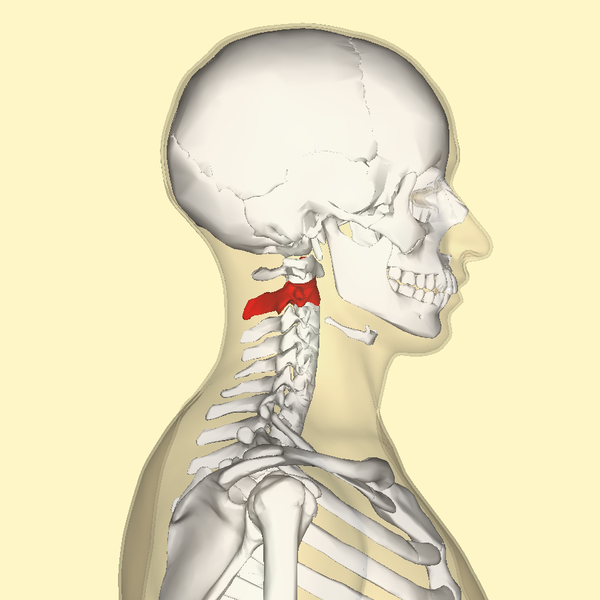
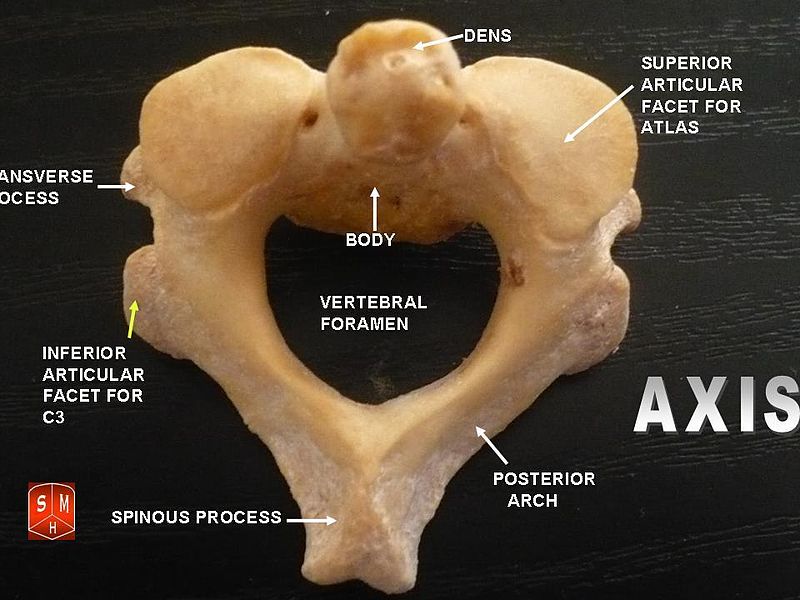
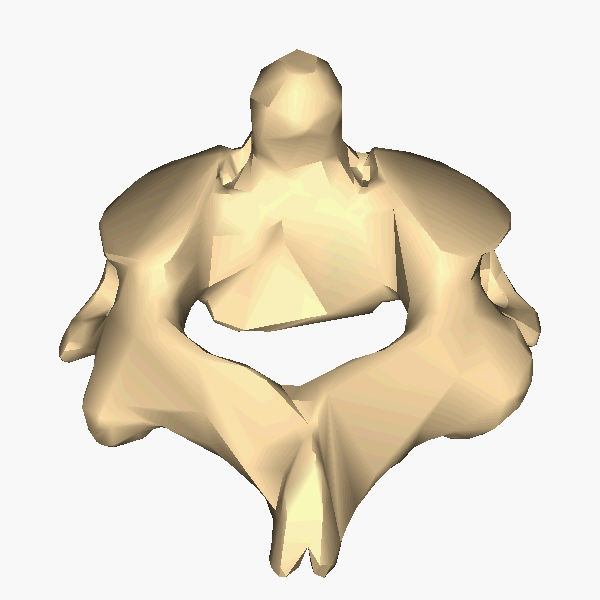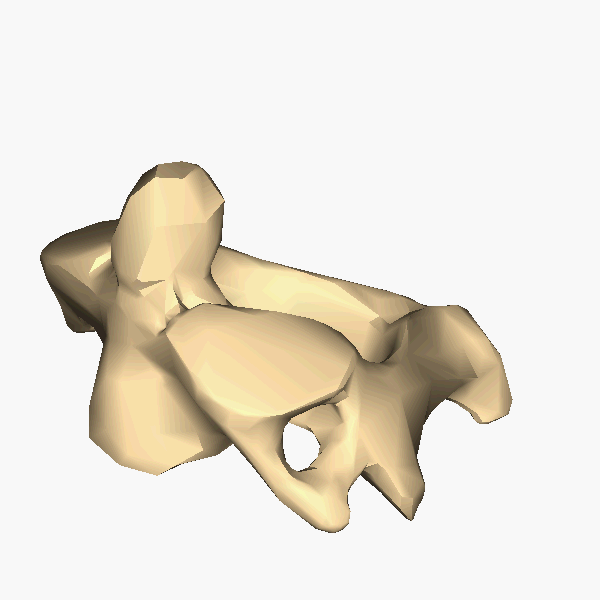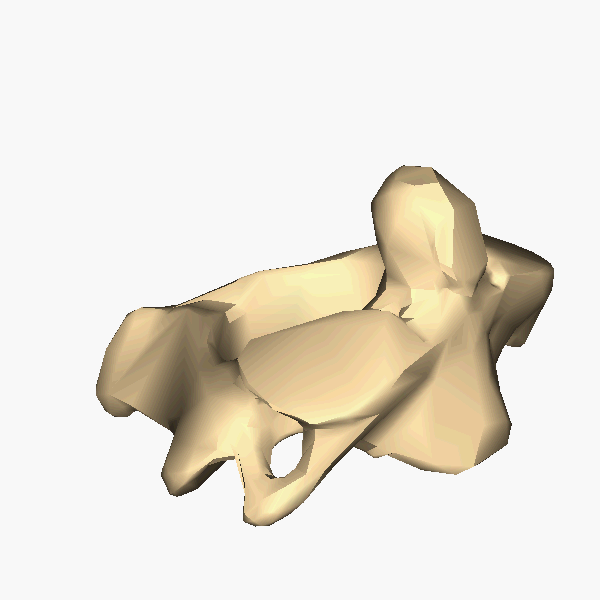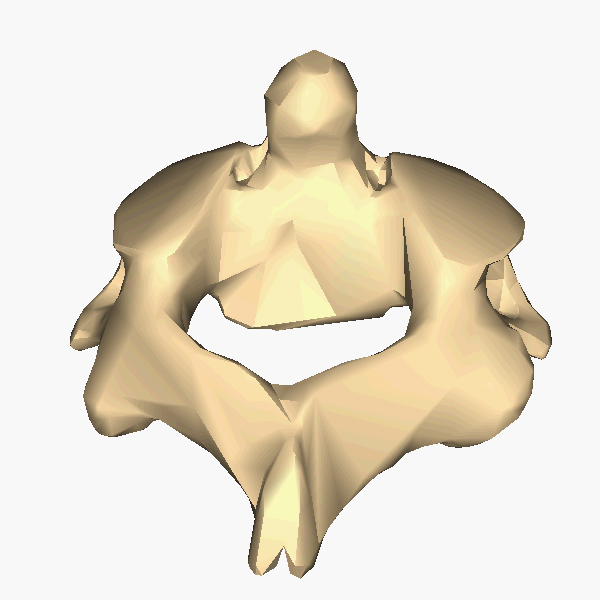
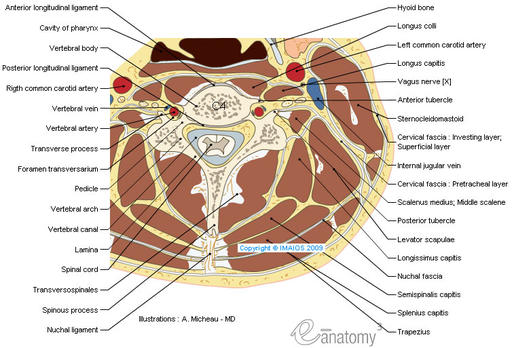
The Atlas-Skull joint controls pitch and the Axis-Atlas joint controls yaw.
Alexander Technique emphasizes gaining an awareness of these motions.
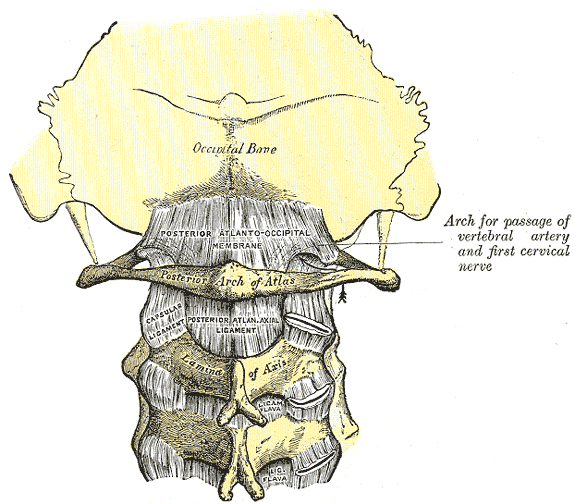 |
 |
|---|---|
 |
|  |
|---|---|---|
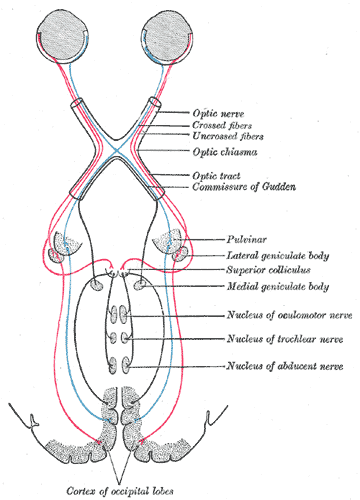
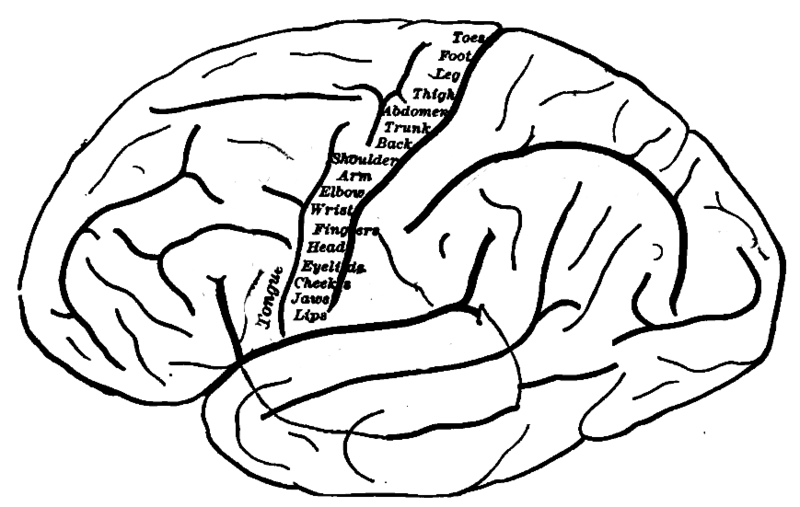
Visual information passes through the motor cortex before being combined at the rear of the brain. The brain and body are an image stabilization system for the eyes.
 |
|---|
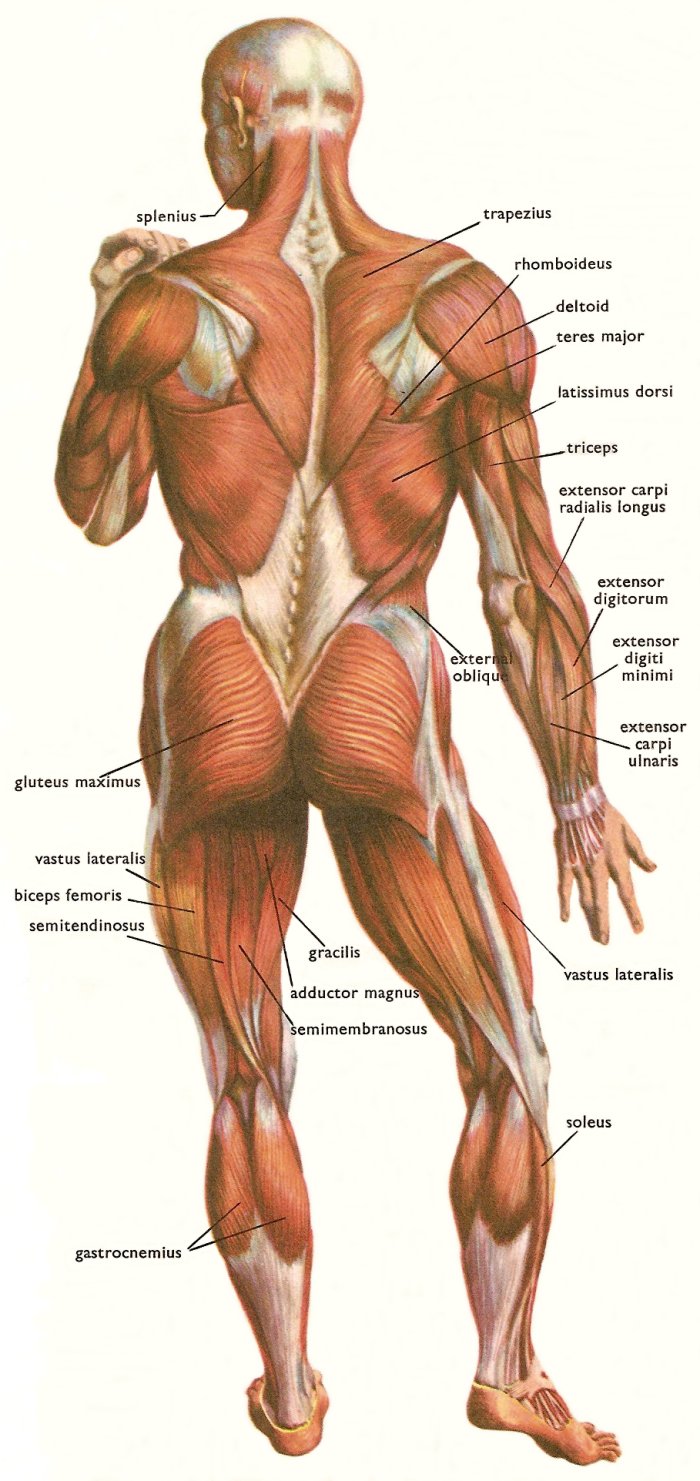
The muscles of the back are continuously connected between the shoulder blades and the hips, to coordinate motion of the limbs.
Bruce Lee: "Balance is the all-important factor in a fighter's attitude or stance. Without balance at all times, he can never be effective."
Jascha Heifetz masterclass In this clip, at time=5:45-6:15, Heifetz emphasizes the importance of balance and posture.
* Marie Daniels illustrates the importance of balance for playing the viola.
Balance flows from a stance with knees in and heels out.
* Gordon Liu demonstrates the wire style at time=4:37
Bruce Lee: "One should seek good balance in motion and not in stillness."
Bruce Lee: "Balance is the control of one's center of gravity plus the control and utilization of body slants and unstable equilibrium, hence gravity pull to facilitate movement. So, balance might mean being able to throw one's center of gravity beyond the base of support, chase it, and never let it get away."
* Jet Li and the Drunken Sword. Try this with a viola bow.
Bruce Lee: "The center of gravity kept under delicate and rapid motion are characteristic habbits of athletes in games that require sudden and frequent changes of direction." Bruce Lee: "The short step and the glide, as contrasted with the hop or cross step, are devices to keep the center of gravity. When it is necessary to move rapidly, a good man takes small enough steps so that his center of gravity is rarely out of control."
Bruce Lee: "In general for athletic contests, a preparatory stance a coiled, or semicrouched posture and a lowered, forward center of gravity. with the bending of the forward knee, the center of gravity moves forward a little. For general readiness, the lead heal usually remains just touching even after the knees bend. Slight ground contact of the heel aids in balance and decreases tension."
Bruce Lee: Experiments indicate that auditory cues, when occurring close to the athlete, are responded to more quickly than visual ones. Make use of auditory clues together with visual clues, if possible. Remember, however, the focus of attention on general movement produces faster action than focus on hearing or seeing the cue.
Time in milliseconds:
.000003 Time for light to cross a 10 meter orchestra
.2 Electric synapse. These synapses are 2-way and they do not amplify signals
2 Chemical synapse. These synapses are 1-way and they can amplify signals
1 Time for a neural signal to travel 10 cm, the size of a brain
10 Time for a neural signal to travel from your fingers to your brain
3 Time for sound to travel 1 meter, the distance to an adjacent musician
7 Period of a 130 Hertz wave. This is the frequency of the viola C string
30 Time for sound to travel across a 10 meter orchestra.
62 Time between notes in "Flight of the Bumblebee"
For an orchestra to have good timing it must use visual cues. Sound isn't fast enough.
This is especially true at the rear of the viola section amidst the cacophony
of winds and brass.
 |
|---|
-776 First Olympic games
-648 The sport of "Pankration" is introduced in the Olympic Games. Similar to MMA.
-536 -520 Milo of Croton dominates Olympic wrestling
-450 Gautama Buddha develops the art of meditation
464 Batuo, a monk from India, founds the Shaolin Temple
500 Bodhidharma, a Buddhist monk, teaches at the Shaolin temple
1600 Emergence of sumo in Japan
~1700 Shaolin temple destroyed by the Chinese Emperor.
The monks who escaped spread Shaolin kung fu throughout China. These monks were:
Ji Sin - Developed Tiger Crane style
Ng Mui - Developed Wing Chun
Bak Mei - Known as "Pai Mei" in kung fu films. Appears in "Kill Bill"
~1700 Fong Sai Yuk. Portrayed by Jet Li in the "Fong Sai Yuk" film series.
1847 1924 Wong Fei Hung. Master of Hung Gar style. Portrayed by Jet Li in
the "Once Upon a Time in China" film series.
1860 1938 Jigoro Kano. Developed Judo and taught it to Mitsuyo Maeda and Moshe Feldenkrais
1868 1910 Huo Yuanjia. Portrayed by Jet Li in the film "Fearless"
1869 1955 F.M. Alexander. Developed "Alexander Technique"
1893 1972 Yip Man. Practitioner of Wing Chun. Teacher of Bruce Lee
1904 1984 Moshe Feldenkrais. Developed "Feldenkrais Technique"
1917 Mitsuyo Maeda teaches Judo to the Gracie family.
Helio Gracie subsequently develops Brasilian Jiu Jitsu
1933 Jigoro Kano trains Feldenkrais in Judo
1940 1973 Bruce Lee
1940 Chuck Norris
1951 Masahiko Kimura vs. Helio Gracie
1952 Sammo Hung
1954 Jackie Chan
1955 Gordon Liu
1963 Jet Li
1963 Michelle Yeoh
1963 Donnie Yen
1970 Shaw Brothers Studios begins mass-producing kung fu films
1993 Age of Mixed Martial Arts begins when Royce Gracie wins a tournament
consisting of fighers with diverse styles.
2000 Kazushi Sakuraba vs. Royce Gracie
2009- Ben Askren dominates MMA with wrestling
2012 Miesha Tate vs. Ronda Rousey
2013 Ben Askren signs with One FC. UFC declines in significance
Interviewer: Other than wrestling what would you say the best base to be a
successful MMA fighter would be?
Ben Askren: Wrestling
 |
 |
 |
|---|---|---|
 |
 |
 |
 |
 |
|---|
 |
 |
|---|
_-_The_Alien.jpg) |
 |
|
|---|---|---|
 |
 |
 |
 |
|---|---|---|---|
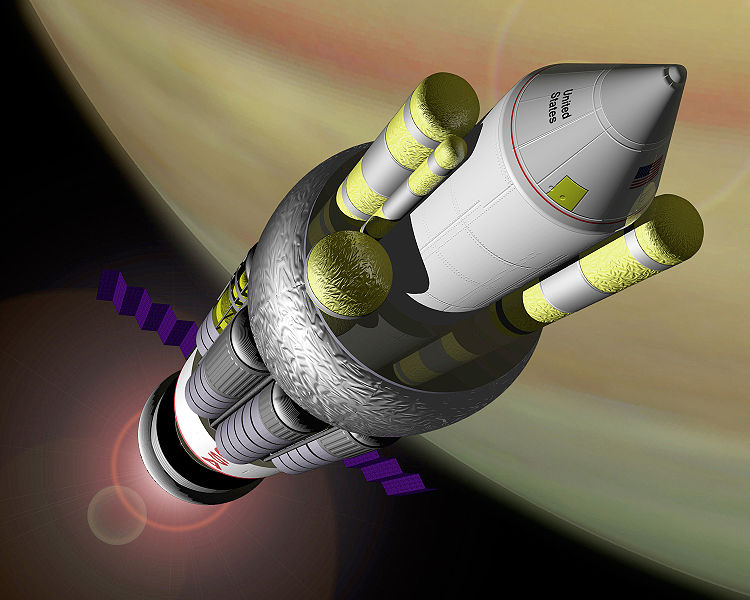
 |
|---|
Rocket propulsion system Exhaust speed
(km/s)
Hydrogen+oxygen rocket 4.4
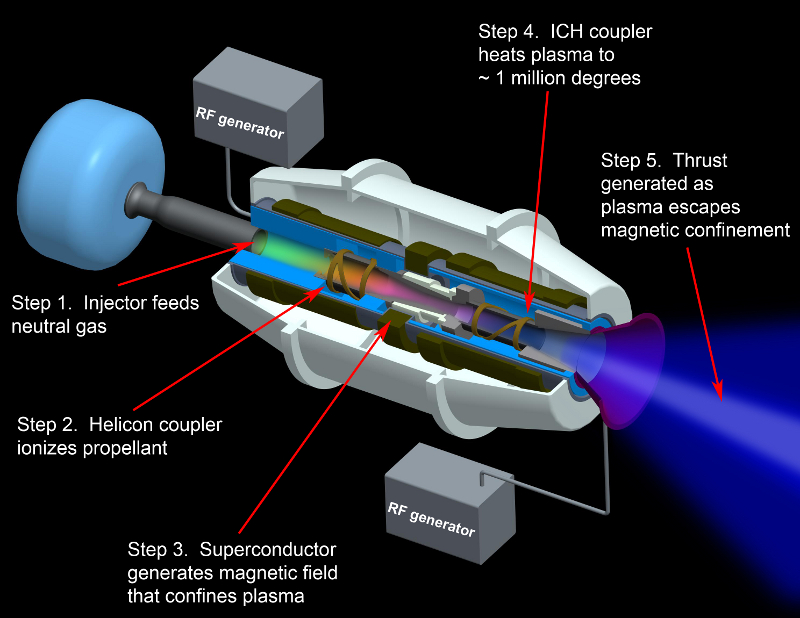
Chang-Diaz ion drive 50 VASIMR design
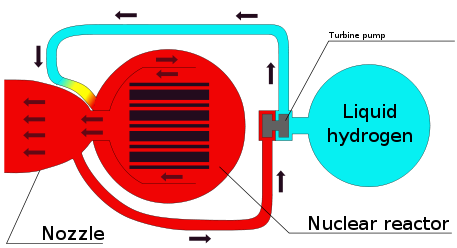
Nuclear thermal rocket, H2 exhaust 9
Orion fusion rocket 10000
Antimatter rocket 1/2 C
All of these rockets are possible with current technology except for the
antimatter rocket.
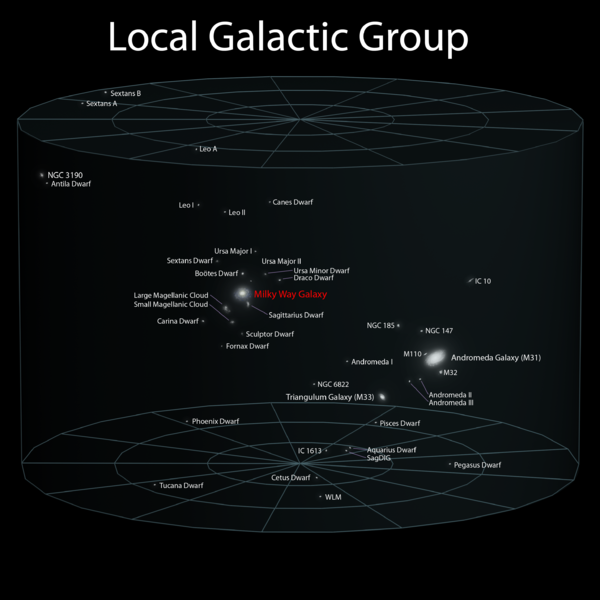 |
|---|
Distance to Alpha Centauri, the nearest star 4.3 light years Milky Way diameter 0.1 million light years Distance to Andromeda 2.5 million light years Distance to the Virgo Supercluster 54 million light years Light travel time during age of universe 13750 million light years Age of the universe ~ 13.75 billion years Age of the Earth ~ 4.54 billion yearsAn alien civilization in the Virgo Supercluster with a 1 billion year head start on us has plenty of time to get here.
 |
|---|
1 2 3 4 3 2 1 0
Hydrogen Helium
Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon
Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
To be a part of a chain, an atom needs at least 2 valence sites and it needs to
be able to form strong bonds.
Single Double Triple Quadruple
B B 3.04
B C 3.69
B O 5.56
C C 3.65 6.45 8.68 6.32
C N 3.19 6.38 9.19
C O 3.73 7.7 11.11
C Si 3.30
C P 2.74
C S 2.82 5.94
N N 1.76 4.33 9.79
N O 2.08 6.29
N Si 3.70
N P
N S
O O 1.50 5.15
O Si 4.69
O P 3.47 5.64
O S 5.41
Si Si 2.30
Si S 3.04
Si P
P P 2.08
P S 3.47
S S 2.34 4.41
H H 4.52
H C 4.25
H N 4.05
H O 3.79
H F 5.89
H Si 3.30
H P 3.34
H S 3.76
H Cl 4.48
96.47 kJoules/mol = 1 eV
Oxygen atmosphere
Fats and sugars for fuel
We'll be able to eat each other's food
As large as or larger than us
Flight is likely
Come from a small planet
Star is less hot than the sun
The planet is volcanic
The planet has a magnetic field
The planet has high metallicity
If a planet is close enough to a star it becomes tidally locked to the star. Mercury is just barely close enough for this and it orbits such that
3 * Orbit period = 2 * Spin period
If it were any closer it would be forced into a lock such that
Orbit period = Spin period
All of the solar system's moons are locked to their planets in this way. None of the planets are locked to their moons except Pluto.
For a low-mass star, the habitable zone is closer to the star than it is for the sun. If the star is sufficiently small, a planet in the habitable zone will be close enough to be locked to the star and will experience extreme weather.
Let L = Luminosity of a star / Luminosity of the sun (Watts). If a star is such that the habitable zone is at Mercury's orbit, what is L? What stellar mass corresponds to this luminosity?
Among the elements required for life, nitrogen is the scarcest.
The nitrogen in the first 250 km of the Earth's crust has the same mass as
the nitrogen in the atmosphere.
The elements that are abundant in the crust and never used by life are aluminum and titanium.
All elements necessary for life are abundant in either the crust, the ocean, or the atmosphere.
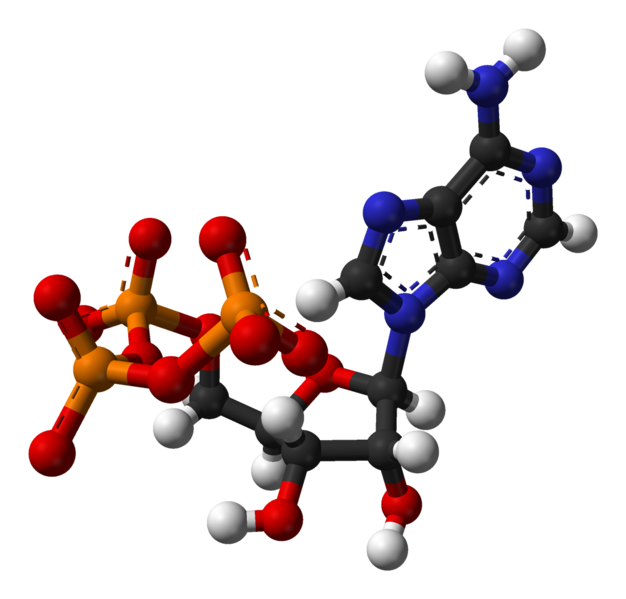
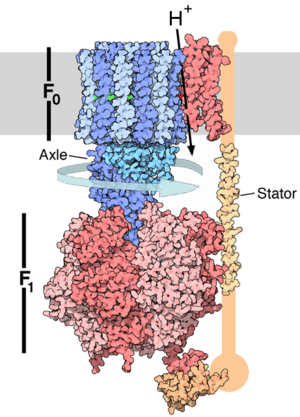
Enzymes use ATP as an energy source to power chemical reactions. ATP and ATP
synthase are common to all Earth life.
* Video of the ATP synthase enzyme in action
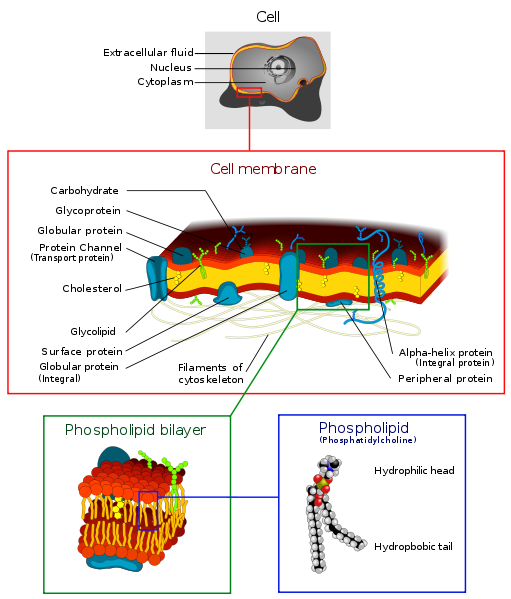
Cell walls are formed from a double layer of lipids. They are elastic and they
self-assemble.
Each lipid has a polar and a non-polar end. The polar end faces the water
and the non-polar end faces another lipid.
* Video of the self-assembly of a bilipid layer
If life were to exist in a non-polar solvent it would have to find another way
to make cell walls.
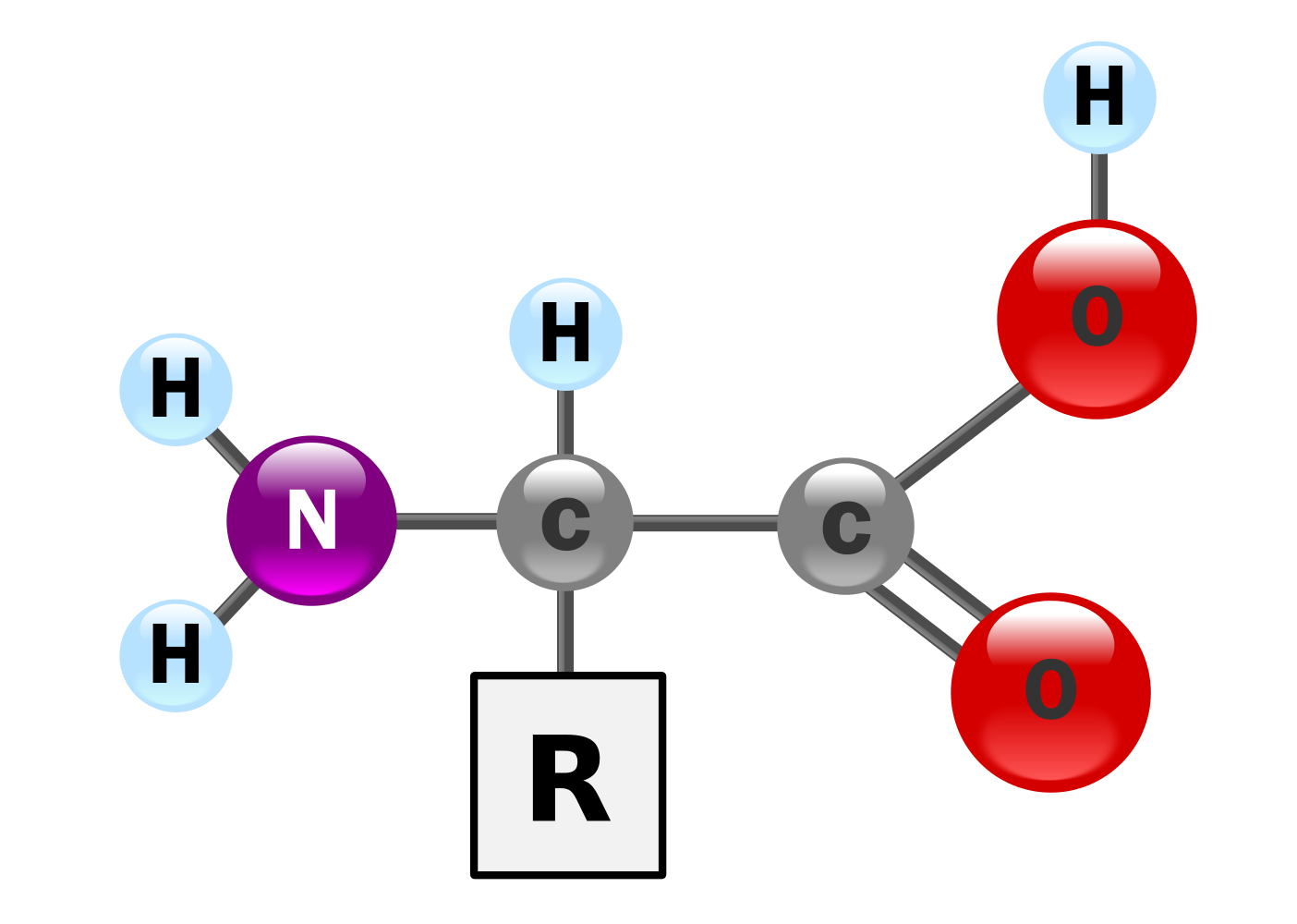
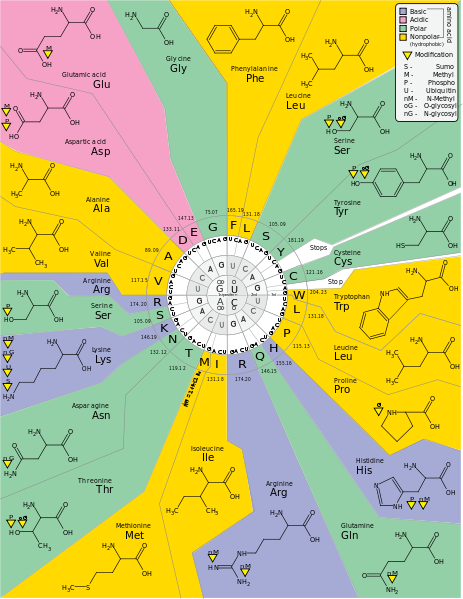
Amino acids have the above form, where R stands for an arbitrary molecule.
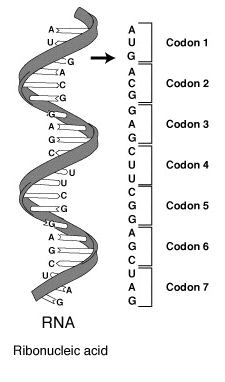
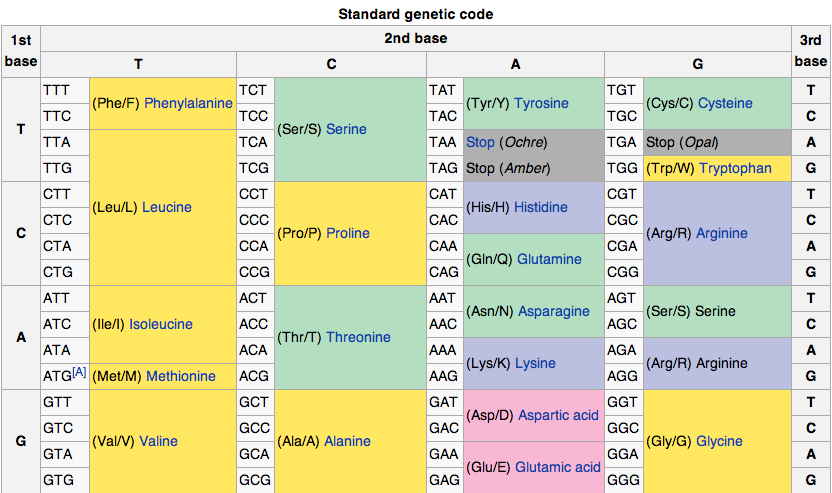
DNA codes a sequence of amino acids. The 64-element codon system is universal to
Earth life.
The codon ATG both codes for methionine and serves as an initiation site: the
first ATG in an mRNA's coding region is where translation into protein begins.
21 amino acids are used by eucaryote. More than 500 amino acids are known.
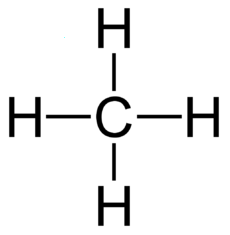
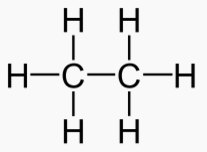
Hydrocarbons have good energy/mass and are good for energy storage. Sugars and fats
are convenient hydrocarbons to metabolize, and humans can metabolize most of them.
An "Alkane" is a carbon chain with hydrocarbons attached. At standard
temperature (300 K), alkanes are solid if they have more than 20 carbons. This
is why lipids (long alkanes) are the optimal form of energy storage. Short
alkanes are liquids or gases at STP and are hard to store.
In the following table, the first section shows properties of alkanes and the second section
shows properties of other energy sources.
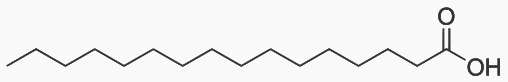
An alkane with 7 or more carbons has a heat of combustion of 46 MJoules/kg.
A nitrogen molecule is more tightly bound than an oxygen molecule, making it impossible
to extract energy from hydrocarbons with nitrogen. Few things burn in a nitrogen atmosphere,
lithium and magnesium being examples.
A sugar generally has the formula CN H2N ON, where
N = 2, 3, etc. The common sugars are hexoses with N=6.
Each sugar molecule has two mirror-symmetric forms, the "D" and "L" form. Only the D forms
are found in nature.
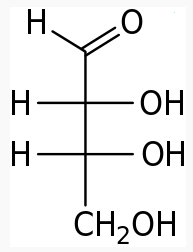
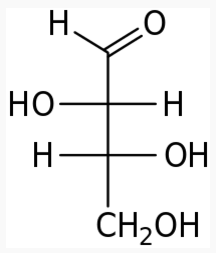
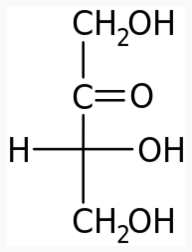
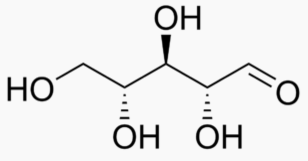
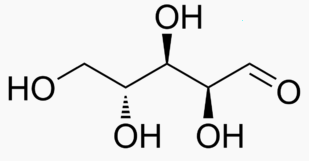
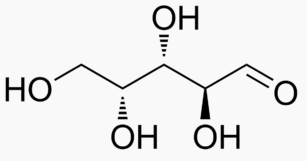
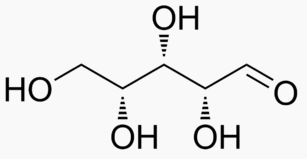
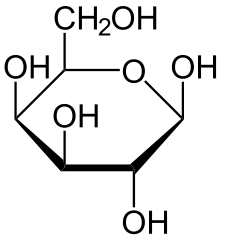
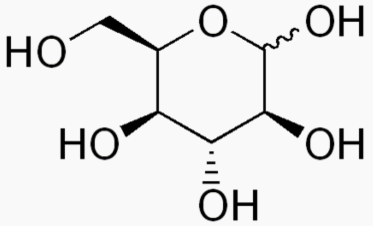
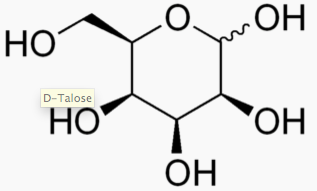
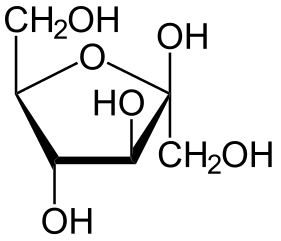
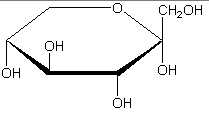
The following figures show all sugars up to 6 carbons. All can be metabolized by humans.
2 carbons:
3 carbons:
4 carbons:
5 carbons:
6 carbons:
Fatty acids and sugars are metabolized in the following stages, with each stage yielding energy.
Blood delivers fatty acids to cells.
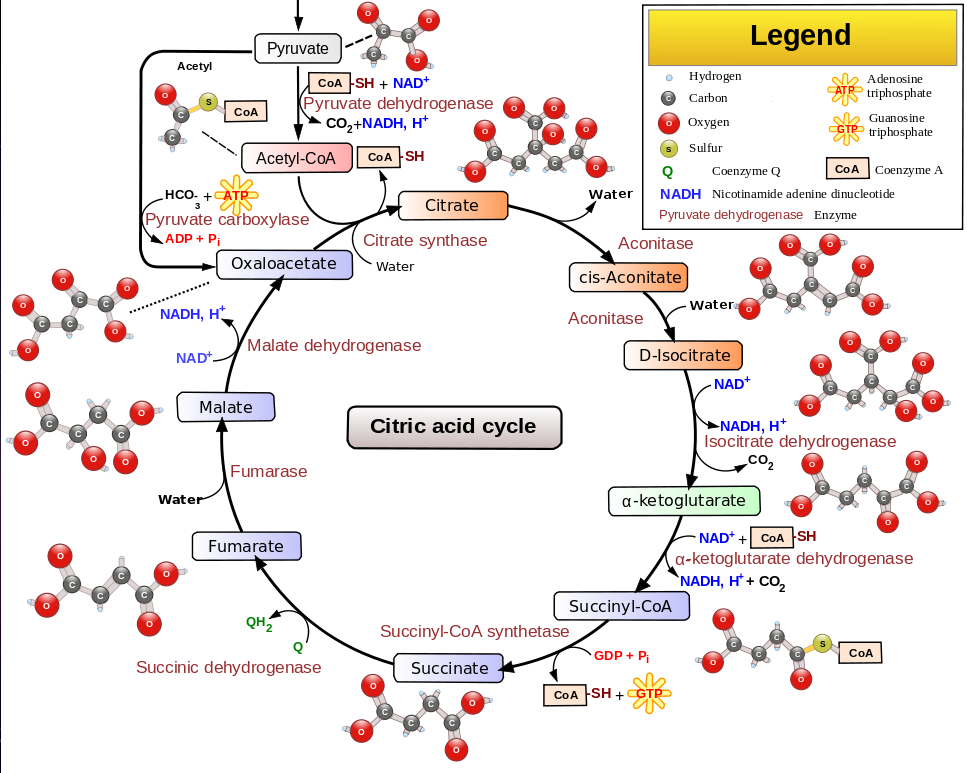
The citric acid cycle (Krebs cycle) converts acetyl or pyrovate into H2O and CO2.
Coenzyme-A carries the acetyl around.
A fat molecule is converted into a fatty acid by lipolysis, and then the fatty acid is converted
into acetyl by beta oxydation, and then the acetyl is converted into H2O and CO2
by the citric acid cycle.
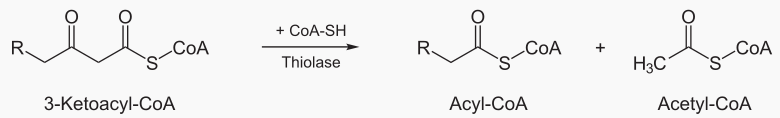
Beta oxidation cleaves 2 carbons from a fatty acid, which becomes acetyl. This process is repeated
until te entire fatty acid has been converted into acetyls.
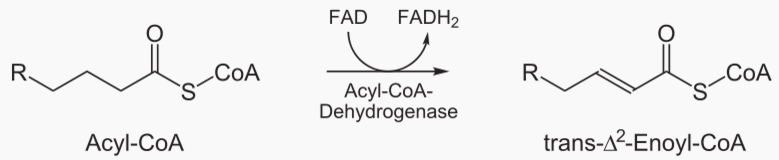
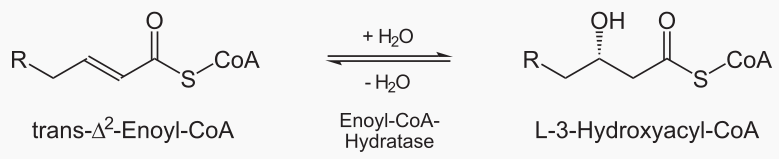
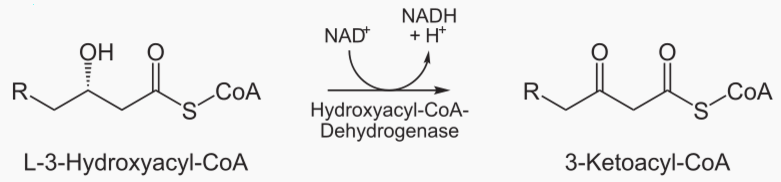
The steps of beta oxidation are:
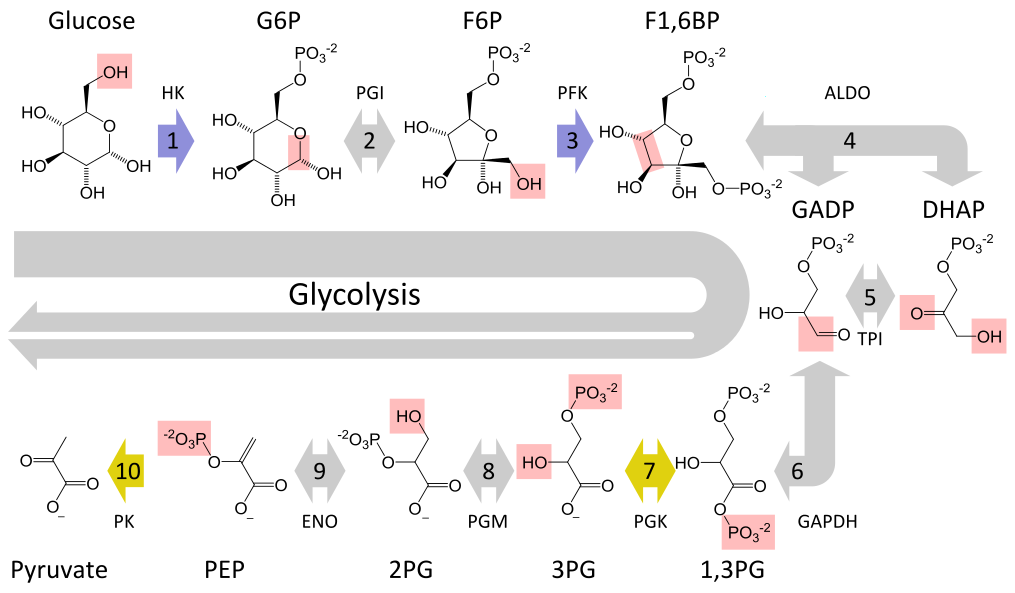
Glycolysis converts a glucose molecule into 2 pyrovate molecules. A summary of the reaction showing only
the starting and ending points is:
The full reaction is:
The citric acid cycle (Krebs cycle) converts acetyl or pyrovate into H2O and CO2.
Fat metabolism oxidizes a carbon chain so that the chain can be split into acetyl.
The strategy of the citric acid cycle is to further oxidize the acetyl (now a part of citrate)
so that the remaining carbon bonds in the acetyl can be broken.
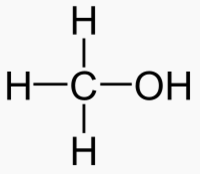
An alcohol is a carbon chain with one OH attached.
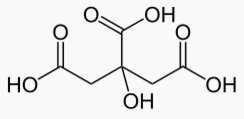
Palmitic acid has 16 carbons and is the most common fatty acid found in food.
Weakly toxic:
Minimum Used by Used Human Crust Ocean Atmosphere
for life humans by life ppt ppt ppt ppt
Hydrogen * * * .10 1.5 108 .00055
Helium .000008 .0052
Lithium .02
Beryllium .0028
Boron * * .0000007 .01
Carbon * * * .18 1.0 .028 .407
Nitrogen * * * .03 .02 780
Oxygen * * * .65 460 858 210
Fluorine * .5
Neon .0000051 .018
Sodium * * * .0015 25 10.8
Magnesium * * * .0005 25 1.3
Aluminum 82
Silicon * 275
Phosphorus * * * .011 1.1
Sulfur * * * .0025 .4 .91
Chlorine * * * .0015 .2 19
Argon .0035 9.3
Potassium * * * .0025 20 .4
Calcium * * * .014 45 .4
Scandium .022
Titanium 5.6
Vanadium * .12
Chromium .10
Manganese * * .00000017 .95
Iron * * .00006 60
Cobalt * * .000000021 .025
Nickel * .084
Copper * * .000001 .06
Krypton .0011
Zinc * * .000032 .075
Gallium .019
Germanium .0015
Arsenic * .0018
Selenium * * .00000019 .00005
Bromine * * .0000029 .0024 .067
Molybdenum * .0012
Tellurium * .000001
Iodine * * .00000016 .00045
Tungsten * .0012
* Video of an amoeba
Synthesis of two amino acids. Proteins are chains of animo acids with a backbone of
the form:
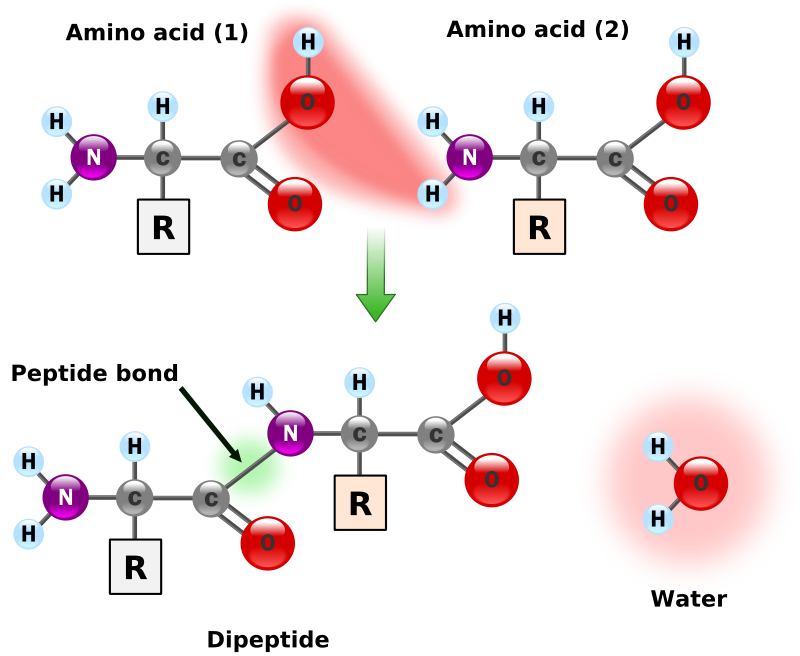
C-C-N-C-C-N-C-C-N-C-C-N-C-C-N
MJoule/kg Calories/gram
Sugar 17 5
Protein 17 5
Alcohol 25 7
Fat 38 9
ATP .057
Phosphocreatine .137
Hydrogen 143
Natural gas 53.6
Gasoline 47
Coal 24
Wood 16
Li-ion battery .6

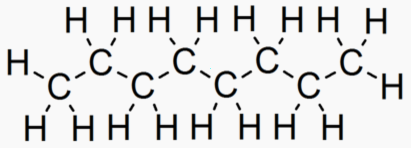
Alkane Carbons Energy of Melt Boil Solid Liquid Gas Phase at
type combustion (K) (K) density density density 300 K
(MJ/kg) (g/cm^3) (g/cm^3) (g/cm^3)
Hydrogen 0 141.8 14.0 20.3 .07 .000090 Gas
Methane 1 55.5 90.7 111.7 .423 .00070 Gas
Ethane 2 51.9 90.4 184.6 .545 .0013 Gas
Propane 3 50.4 85.5 231.1 .60 .0020 Gas
Butane 4 49.5 136 274 .60 .0025 Gas
Pentane 5 48.6 143.5 309 .63 Liquid
Hexane 6 48.2 178 342 .65 Liquid
Heptane 7 48.0 182.6 371.5 .68 Liquid
Octane 8 47.8 216.3 398.7 .70 Liquid
Dodecain 12 46 263.5 489 .75 Liquid
Hexadecane 16 46 291 560 .77 Liquid
Icosane 20 46 310 616 .79 Solid
Alkane-30 30 46 339 723 .81 Solid
Alkane-40 40 46 355 798 .82 Solid
Alkane-50 50 46 364 848 .82 Solid
Alkane-60 60 46 373 898 .83 Solid
Gasoline ~ 8 47 .76 Liquid Mostly alkanes with ~ 8 carbons
Natural gas 54 91 112 Gas Mostly methane
Coal 32 - - Solid Mostly carbon
Wood 22 - - Solid Carbon, oxygen, hydrogen
Pure carbon 1 32.8 - - Solid Pure carbon, similar to coal
Methanol 1 175.6 337.8 .79 Liquid
Ethanol 2 159 351.5 .79 Liquid
Propanol 3 147 370 Liquid
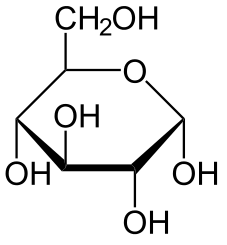
Number of Number of
carbons sugars
Diose 2 1
Triose 3 2
Tetrose 4 3
Pentose 5 4
Hexose 6 12 At least 6 carbons are required to form a ring
Heptose 7 many Rarely observed in nature
Octose 8 many Unstable. Not observered in nature.
"Number of sugars" refers to the number of different types of sugar molecules for each
carbon number.
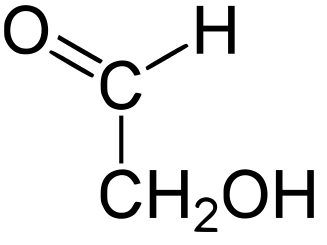
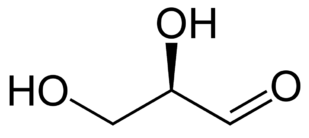
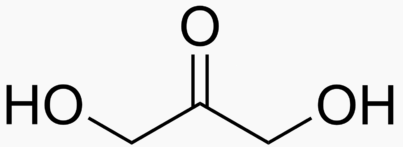

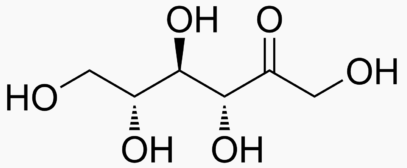
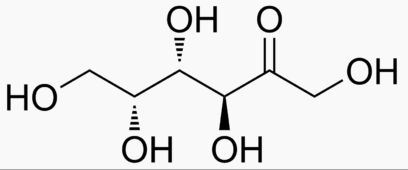
Energy Sweetness
Succrose 1.00 1.00 Benchmark
Glucose .74
Maltose .32
Galactose .32
Lactose .16
Allose
Altrose
Mannose
Fructose 1.73
Psichose .70
Tagatose .38 .92
Sorbose 1.0
Honey .97
Monosaccharde: 1 sugar molecule
Disaccharide: 2 monosaccharides
Polysaccharide: More than 2 monosaccharides, such as starch and cellulose
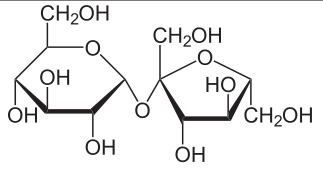
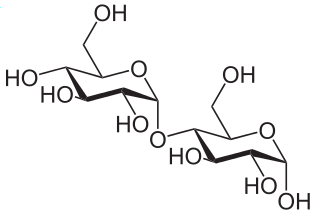
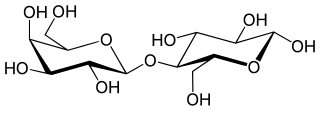
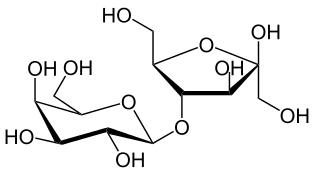
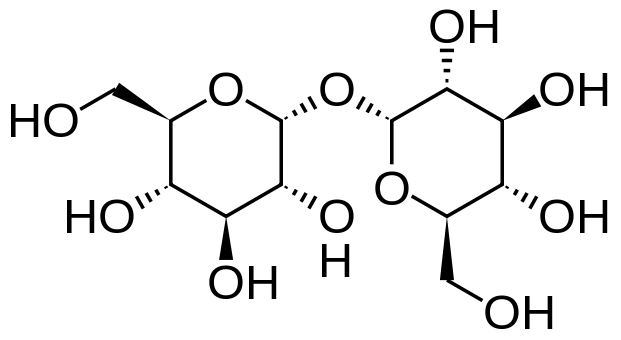
Sucrose = Glucose + Fructose
Maltose = Glucose + Glucose
Lactose = Galactose + Glucose
Lactulose = Galactoce + Fructose
Trehalose = Glucose + Glucose
Cellobiose = Glucose + Glucose
Chitobiose = Glucosamine + Glucosamine
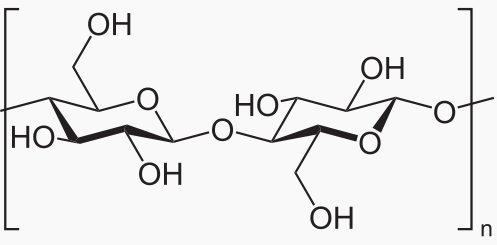
Starch and cellulose are long chains of glucose molecules.
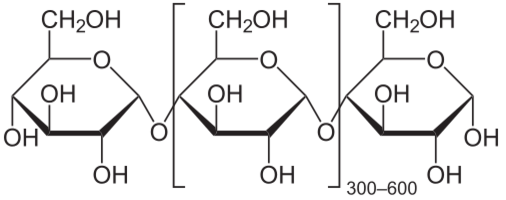
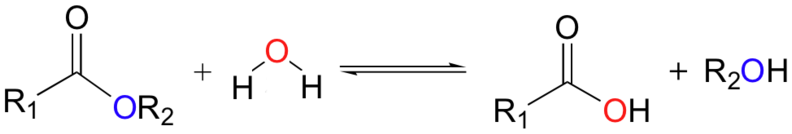
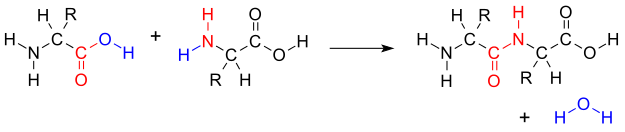

![]()
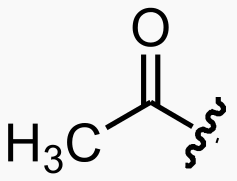
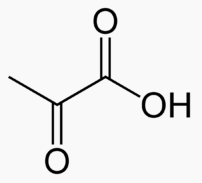
Fatty acid -> Acetyl -> CO2 and H2O
Sugar -> Pyruvate -> CO2 and H2O
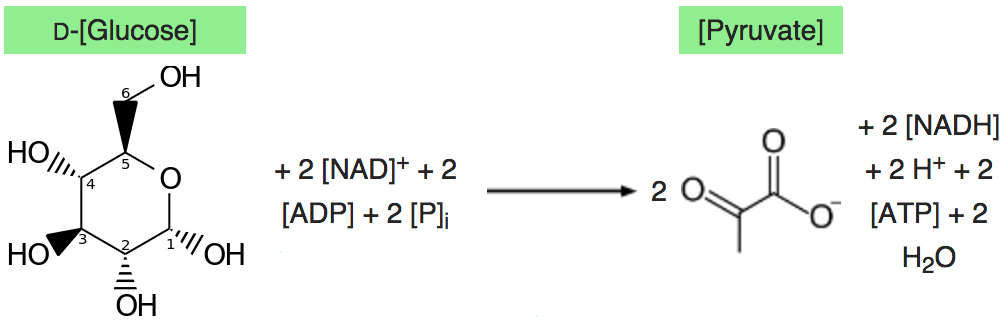
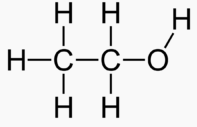
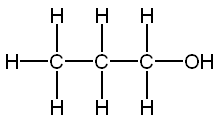
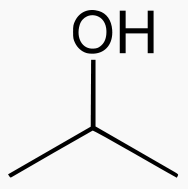
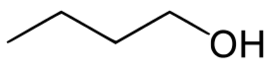
Carbons
Methanol 1 Toxic
Ethanol 2 Inebriating
Propanol 3 3 times more inebriating than ethanol
Isopropanol 3 Toxic
Butanol 4 6 times more inebriating than ethanol
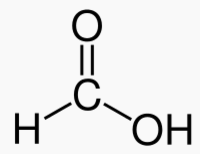
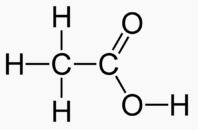

Carbons
1
2 Vinegar
3
4 Found in butter
8 Found in coconuts
10 Found in coconuts
12 Found in coconuts
16 Most common fatty acid. Found in palm oil
18 Found in chocolate
20 Found in peanut oil
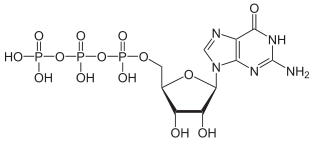
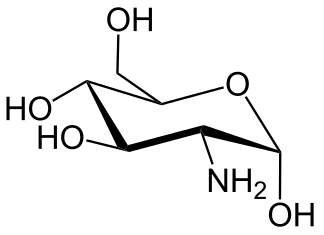


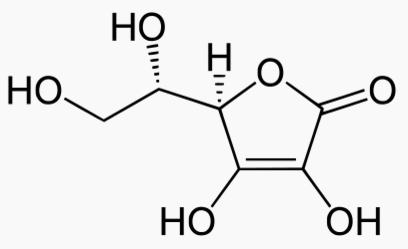
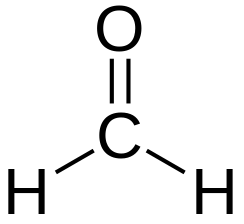
LD50
(mg/kg)
CO Carbon monoxide
HCN 6.4 Hydrogen cyanide
CH2O Methanol
CH2O Formaldehyde
H2S Hydrogen sulfide
NO2 Nitrite
Cl2 Chlorine
Fl2 Fluorine
Ethanol 7060
Salt 3000
Caffeine 192
Aspirin 200
NaNO2 180 Sodium nitrite
Cobalt 80
NaF 52
Capsaicin 47 Chili pepper
Mercury 41
Arsenic 13
Nicotine .8
Bromine
C2N2
PH3
SiCl4
Almost anything with fluorine or bromine is toxic.
C2H2 Acetylene. Inebriating
C3H6 Propene. Inebriating



































































































Hydrogen White
Carbon Black
Nitrogen Blue
Oxygen Red
Sulfur Yellow
Scoville scale (relative capsaicin content)
Ghost pepper 1000000
Trinidad 1000000 Trinidad moruga scorpion
Naga Morich 1000000
Habanero 250000
Cayenne pepper 40000
Malagueta pepper 40000
Tabasco 40000
Jalapeno 5000
Guajillo pepper 5000
Cubanelle 500
Banana pepper 500
Bell pepper 50
Pimento 50
Molecule Relative hotness
Rresiniferatoxin 16000
Tinyatoxin 5300
Capsaicin 16 Chili pepper
Nonivamide 9.2 Chili pepper
Shogaol .16 Ginger
Piperine .1 Black pepper
Gingerol .06 Ginger
Capsiate .016 Chili pepper