




 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
 |
 |
|---|---|---|
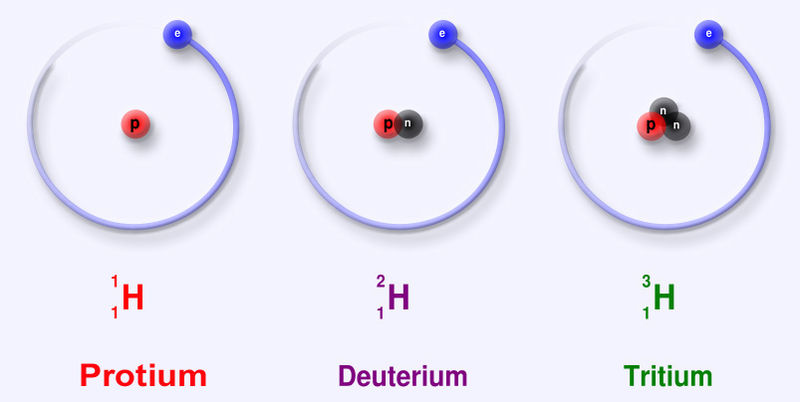
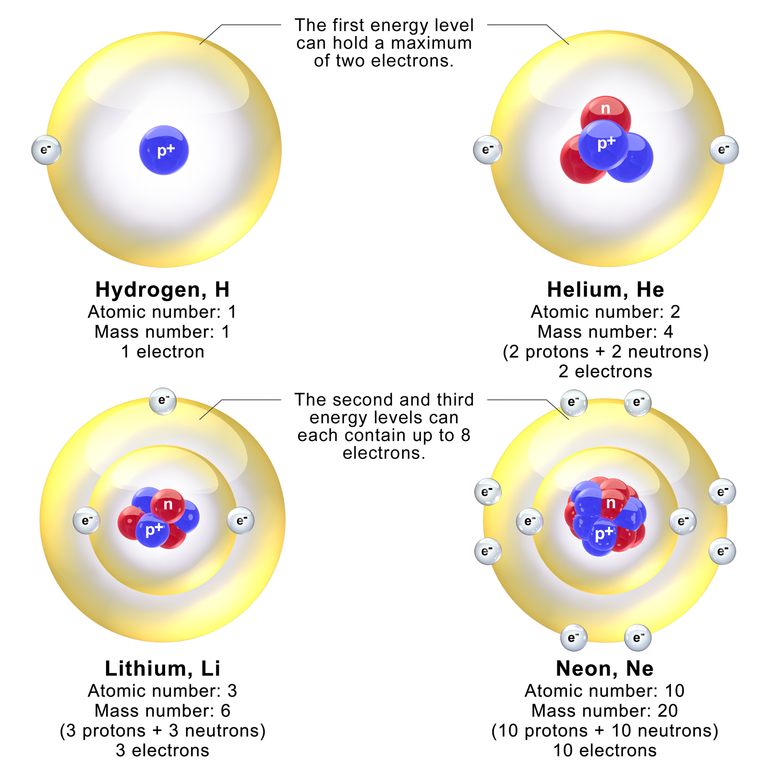
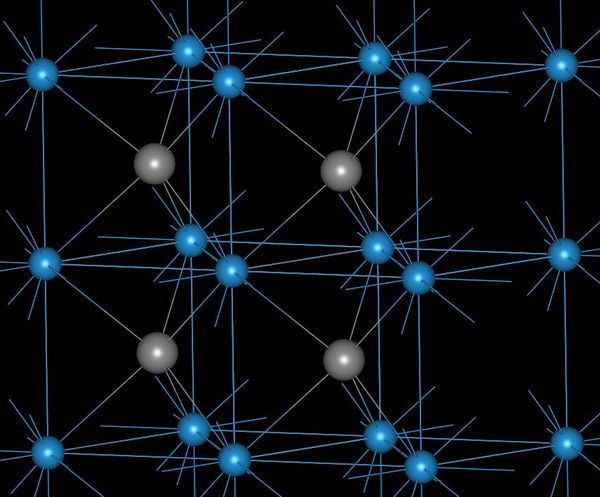
Elements are built from protons, neutrons, and electrons. The identity of an element is determined by the proton number. The heaviest naturally-occuring element is uranium with 92 protons.
Particle Charge Mass (kg) Mass / Proton mass Proton +1 1.673⋅10-27 1 Neutron 0 1.675⋅10-27 1.0012 Electron -1 9.109⋅10-31 .000544
.png) |
|---|
 |
|---|
An element has a fixed number of protons and a variable number of neutrons. Each neutron number corresponds to a different isotope. Naturally-occuring elements tend to be a mix of isotopes.
Isotope Protons Neutrons Natural fraction Hydrogen-1 1 0 .9998 Hydrogen-2 1 1 .0002 Helium-3 2 1 .000002 Helium-4 2 2 .999998 Lithium-6 3 3 .05 Lithium-7 3 4 .95 Beryllium-9 4 5 1 Boron-10 5 5 .20 Boron-11 5 6 .80 Carbon-12 6 6 .989 Carbon-13 6 7 .011Teaching simulation for isotopes at phet.colorado.edu
Atom masses are measured in "atomic mass units" (AMU). One AMU is defined as 1/12 the mass of a carbon-12 atom and is approximately equal to the mass of a hydrogen-1 atom.
Mass (Atomic mass units)
Proton 1.0072765
Neutron 1.0086649
Electron .0005486
Atomic mass unit 1.000000 = 1.6605-27 kg
Hydrogen-1 1.007825
Hydrogen-2 2.014102
Hydrogen-3 3.016049
Hydrogen ave. mass 1.00798
Carbon-12 12.00000
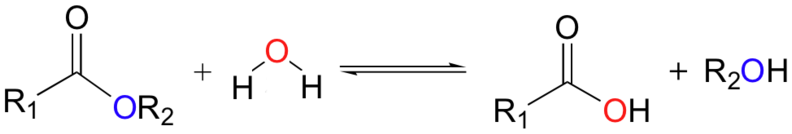
The atomic mass for an element is the average over all isotopes. For hydrogen,
Hydrogen-1 abundance = .9998
Hydrogen-2 abundance = .0002
Hydrogen-3 abundance = 0 (Unstable)
Average mass of hydrogen = (Hydrogen-1 fraction) * (Hydrogen-1 mass) + (Hydrogen-2 fraction) * (Hydrogen-2 mass)
= .9998 * 1.007825 + .0002 * 2.014102
= 1.00798
 |
|---|
For gases, the density at boiling point is used. Size data
 |
|---|
Copper atoms stack like cannonballs. We can calculate the atom size by assuming the atoms are shaped like either cubes or spheres. For copper atoms,
Density = D = 8900 kg/m3 Atomic mass unit= M0 = 1.661⋅10-27 kg Atomic mass = MA = 63.55 Atomic mass units Mass = M = MA⋅M0 = 9.785⋅10-26 kg Number density = N = D / M = 9.096⋅1028 atoms/m3 Cube volume = Υcube= 1 / N = 1.099⋅10-29 m3 Volume/atom if the atoms are cubes Cube length = L = Υ1/3cube = 2.22⋅10-10 m Side length of the cube Sphere fraction = f = π/(3√2) = .7405 Fraction of volume occupied by spheres in a stack o spheres Sphere volume = Υsph= Υcube f = 8.14⋅10-30 m3 = 4⁄3πR3 Volume/atom if the atoms are spheres Sphere radius = R = 1.25⋅10-10 m
 |
|---|
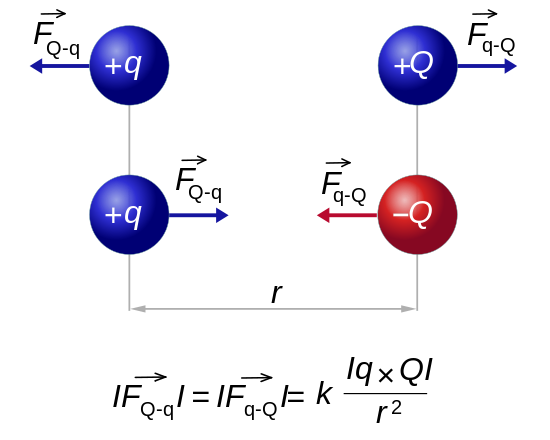
Charge 1 Charge 2 Electric Force + + Repel - - Repel + - Attract - + AttractThe gravitational and electric forces follow the same equations. The fundamental unit of charge is the "Coulomb".
Mass = M Charge = Q (Coulombs) Charge on a proton = Qp = 1.602e-19 Coulombs Distance between charges = R Gravity constant = G = 6.67⋅10-11 Newton m2 / kg2 Electric constant = K = 8.99⋅109 Newton m2 / Coulomb2 Gravity force = Fg = -G M1 M2 / R2 Electric force = Fe = -K Q1 Q2 / R2 Gravity energy = Eg = -G M1 M2 / R Electric energy = Ee = -K Q1 Q2 / R
Energy is measured in either Joules or electron Volts (eV). An electron Volt is the energy gained by an electron upon falling down an electric potential of 1 Volt.
1.6022⋅10-19 Joules = 1 eV Red photon = 1.8 eV Green photon = 2.3 eV Blue photon = 3.1 eV O2 molecule energy = 5.2 eV Energy released when two oxygens combine to O2 Hydrogen ionization = 13.6 eV Energy to remove the electron from hydrogen
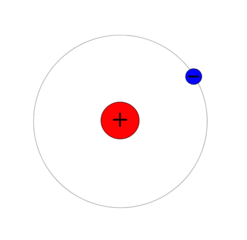
Calculating the energy levels of an electron in a hydrogen atom requires quantum mechanics, but we get useful results by oversimplifying and assuming the electron is on a circular orbit around the proton. Quantum mechanics tells us that the lowest energy level has an orbit radius of 5.29⋅10-11 meters and an energy of -13.6 eV.
1 electron Volt (eV) = 1.60⋅10-19 Joules Unit of energy for particles
Electron mass = Me = 9.11⋅10-31 kg
Electron charge = C = -1.60⋅10-19 Coulombs
Electron orbit radius = R = 5.29⋅10-11 meters "Bohr radius"
Electron orbit speed = V = 2190 km/s = .041 ⋅ Lightspeed
Electric force constant = K = 8.99⋅109 Newtons meters2 / Coulombs2 "Coulomb constant"
Electric force = Fe = -K C2 / R2 Force between the electron and proton
Centripetal force = Fc = M V2 / R
Electric potential energy = Ee = -K C2 / R = -27.2 eV Potential energy between the electron and proton
Electron kinetic energy = Ek = ½ M V2 = +13.6 eV
Electron total energy = Et = Ek + Ee = -13.6 eV = -½ Ee
Electric force = Centripetal force
Fe = Fc
K C2 / R = M V2
-Ee = 2 Ek
This sets the characteristic length and energy of chemical bonds.
Chemical bonds have energies ranging from 2 to 9 eV. Bonds that are weaker
than this tend to be unstable.
 |
 |
|---|---|
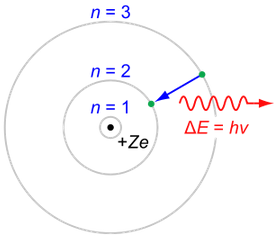
The lowest energy level is -13.6 eV and the higher levels are given by
Energy level = N
= 1 for the lowest energy level
= 2 for the next lowest level, etc.
Energy of lowest level = -Eo = -13.6 eV
Energy of level N = -Eo N-2
Orbit radius of lowest level = Ro = 5.29⋅10-11 meters "Bohr radius"
Orbit radius of level N = Ro N2
The "Bohr model" is an empirical way to calculate these levels. In quantum
mechanics particles have an intrinsic wavelength and an electron orbital is
interpreted as a vibrating string with this wavelength. The allowed wavelengths
correspond to the overtones of the string and the number of wavelengths
experienced by an electron in one orbit must be an integer.
Particle momentum = S = M V particle wavelength = λ Planck constant = h = 6.62⋅10-34 Joule seconds Orbit radius = R Orbit circumference = C De Broglie formula: h = S λ Overtone rule: C = N λ where N is an integer and this determines the orbital type N Orbital 1 S 2 P 3 D 4 FSetting N=1 gives the lowest energy level.
2 π R = λ = h / Q = h / (MV) For a circular orbit, K Q2 / R = M V2 Ro = (h/2π)2 / (K M Q^2) Eo = ½ K2 Q4 M (2π/h)2
 |
|
|---|---|
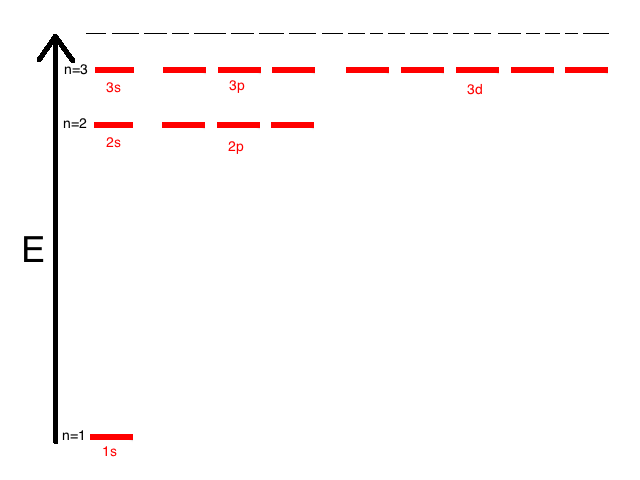
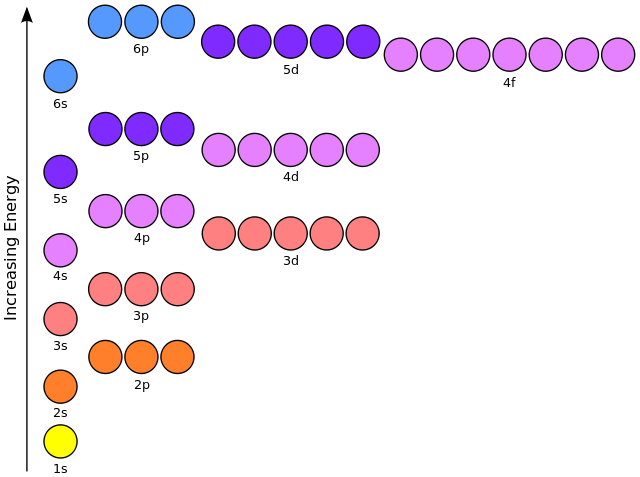
If a nucleus has multiple protons and one electron then the relative spacing between the levels is the same as for a nucleus with one proton. The electron energy levels are
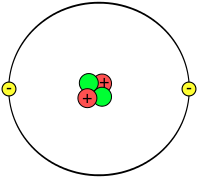
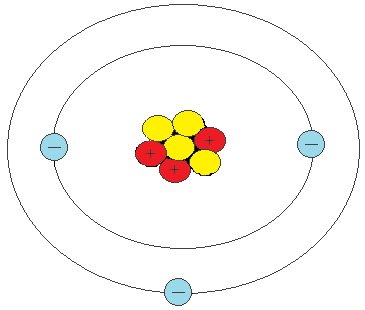
Energy level = N Number of protons = Z Energy of the lowest level = -Eo = -13.6 eV Energy of level N = -Eo Z2 N-2 Orbit radius of lowest level = Ro = 5.29⋅10-11 meters Orbit radius of level N = Ro N2 / ZIf there are multiple electrons then the levels change. The electrons orbiting a nucleus are in discrete energy levels, each represented by a circle in the above figure. Each level can have 0, 1, or 2 electrons and not more. Electrons seek the lowest energy level and if it's occupied they settle in the next-highest energy level.
 |
|---|
Each level can have 0, 1, or 2 electrons and not more. Electrons seek the lowest energy level and if it's occupied they settle in the next-highest energy level.
Orbital Number of Maximum # type levels of electrons S 1 2 P 3 6 D 5 10 F 7 14
_-_electron_orbitals.svg.png) |
|---|
Protons Max S1 S2 P2 S3 P3
bonds
H Hydrogen 1 1 1 0 0 0 0
He Helium 2 0 2 0 0 0 0
Li Lithium 3 1 2 1 0 0 0
Be Beryllium 4 2 2 2 0 0 0
B Boron 5 3 2 2 1 0 0
C Carbon 6 4 2 2 2 0 0
N Nitrogen 7 3 2 2 3 0 0
O Oxygen 8 2 2 2 4 0 0
F Fluorine 9 1 2 2 5 0 0
Ne Neon 10 0 2 2 6 0 0
Na Sodium 11 1 2 2 6 1 0
Mg Magnesium 12 2 2 2 6 2 0
Al Aluminum 13 3 2 2 6 2 1
Si Silicon 14 4 2 2 6 2 2
P Phosphorus 15 3 2 2 6 2 3
S Sulfur 16 2 2 2 6 2 4
Cl Chlorine 17 1 2 2 6 2 5
Ar Argon 18 0 2 2 6 2 6
Fe Iron 26 many 2 2 6 2 6
Au Gold 79 many 2 2 6 2 6
U Uranium 92 many 2 2 6 2 6
Max bonds: Maximum number of chemical bonds that the element can form.
Molecule number is measured in moles, which are defined such that one mole of Carbon-12 atoms has a mass of exactly 12 grams.
Avogadro number = NAvo = 6.022⋅1023 = Number of molecules in one mole One mole of carbon-12 = 12.000 grams (exact) One mole of hydrogen = 1.008 grams = 1.674⋅10-27 kg One mole of water = 18.015 grams = 2.992⋅10-26 kg 1 electron Volt = 1 eV = E0 = 1.6022⋅10-19 Joules Energy per mole = Emol (Joules/mole) Energy per particle in eV = EeV = Emol / NAvo / 1.6022⋅10-19 = 1.0364⋅10-5 Emol = EMol / 96488 (eV)
 |
|---|
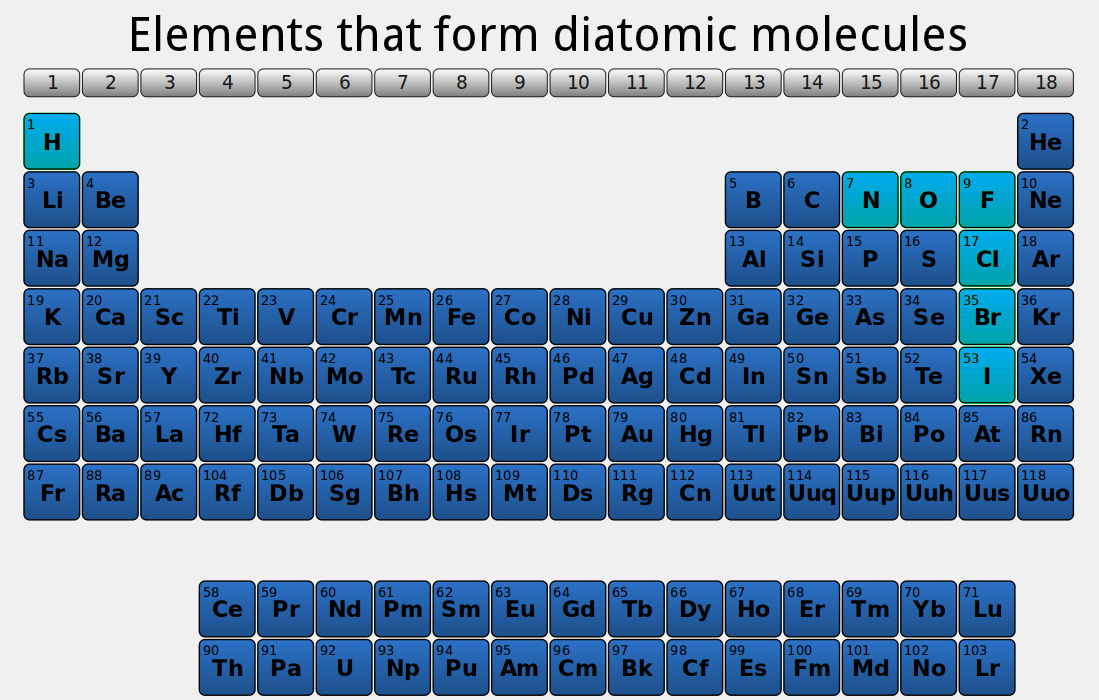
If two atoms of hydrogen encounter each other they combine into a diatomic molecule, releasing energy. All elements that are gases at room temperature are diatomic.
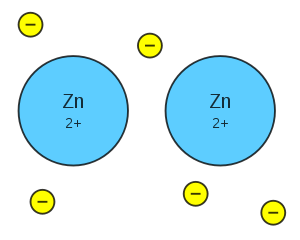 |
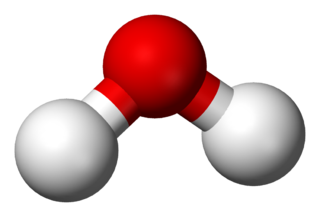 |
 |
|
|---|---|---|---|
For the dissociation of water,
OH + H ↔ H2O + 5.12 eV Remove the first hydrogen from the oxygen O + H ↔ HO + 4.41 eV Remove the second hydrogen from the oxygen O + H + H ↔ 2HO + 2*4.76 eV Remove both hydrogens from the oxygen Water molecule energy = 2*4.76 eV = 5.12 eV + 4.41 eVThe first two rows are "bond dissociation energies" and the last row is a "mean bond energy" (4.76 eV). The mean bond energy is the total energy of the water molecule divided by the number of bonds.
For methane, the average bond energy is 4.31 eV.
CH3 + H ↔ CH4 + 4.52 eV CH2 + H ↔ CH3 + 4.61 eV CH + H ↔ CH2 + 4.61 eV C + H ↔ CH + 3.52 eV C + H + H + H + H ↔ CH4 + 4*4.31 eV
Hydrogen forms molecules with all elements except the noble gases, osmium,
iridium, promethium, francium, and radium. This makes it a benchmark for
determining the number of bonds that each element forms, as well as the
strength of each element's attraction for electrons.
The "valence number" is the number of bonds that an element can form. The
valence number for each element can be inferred from the table of hydrides.
Elements in the same column of the periodic table have the same valence number.
The following table shows the valence number for each column of the periodic table.
The table gives the most common oxidation number for each metal.
The oxides of copper are:
The oxides of iron are:
In the table, the "e-" column denotes the number of electrons
given by the element to oxygen atoms.
Every atom attracts electrons and the electronegativity table shows the
relative energy released when the atom captures an electron. The elements in
the upper right are the most electron-hungry.
In the reaction H + H + O → H2O,
The oxygen steals an electron from each of the two hydrogens. It is able to do this
because the electrons are at a lower energy with oxygen than with hydrogen.
Most chemical reactions involve elements on the left side of the periodic table
giving electrons to elements on the right side.
The table gives the energy to remove a hydrogen from a hydride molecule.
This is the data used to construct the electronegativity table.
For a system in thermodynamic equilibrium each degree of freedom has
a mean energy of ½ k T. This is the definition of temperature.
The sound speed is proportional to the thermal speed of gas molecules. The
thermal speed of a gas molecule is defined in terms of the mean energy per
molecule.
For air, γ = 7/5 and
We can change the sound speed by using a gas with a different value of M.
In a gas, some of the energy is in motion of the molecule and some is in
rotations and vibrations. This determines the adiabatic constant.
The fact that Newton's calculation differed from the measured speed is due to
the fact that air consists of diatomic molecules (nitrogen and oxygen). This
was the first solid clue for the existence of atoms, and it also contained a
clue for quantum mechanics.
In Newton's time it was not known that changing the volume of a gas changes its
temperature, which modifies the relationship between density and pressure.
This was discovered by Charles in 1802 (Charles' law).
Carbon dioxide doesn't have a liquid state at standard temperature and pressure.
It sublimes directly from a solid to a vapor.
For an object to have an atmosphere, the thermal energy must be much less than the
escape energy.
The threshold for capturing an atmosphere appears to be around Z = 1/25, or
When an object collapses by gravity, its temperature increases such that
For an ideal gas,
The Earth's core is composed chiefly of iron. What is the temperature of an iron
atom moving at the Earth's escape speed?
We first derive the law for a 1D gas and then extend it to 3D.
Suppose a gas molecule bounces back and forth between two walls separated
by a distance L.
Time between collisions = 2 L / V
The average force on a wall is
A typical globular cluster consists of millions of stars.
If you measure the total gravitational and kinetic energy of the stars, you will
find that
Suppose a system consists of a set of objects interacting by a potential. If
the system has reached a long-term equilibrium then the above statement about
energies is true, no matter how chaotic the orbits of the objects. This is the
"Virial theorem". It also applies if additional forces are involved. For
example, the protons in the sun interact by both gravity and collisions and the
virial theorem holds.
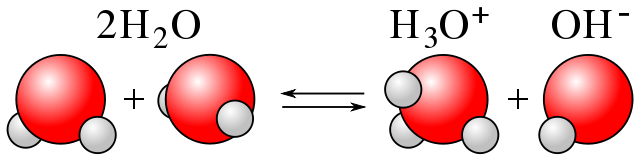
For an acid dissolved in water,
A molecule is organic if it contains carbon. Molecules are often depicted with
the hydrogens excluded.
An "Alkane" is a carbon chain with hydrocarbons attached. At standard
temperature (300 K), alkanes are solid if they have more than 20 carbons. This
is why lipids (long alkanes) are the optimal form of energy storage. Short
alkanes are liquids or gases at STP and are hard to store.
In the following table, the first section shows properties of alkanes and the
second section shows properties of other energy sources.
An alkane with 7 or more carbons has a heat of combustion of 46 MJoules/kg.
A nitrogen molecule is more tightly bound than an oxygen molecule, making it
impossible to extract energy from hydrocarbons with nitrogen. Few things burn
in a nitrogen atmosphere, lithium and magnesium being examples.
A chain is "saturated" if it contains the maximum number of hydrogen atoms
and "unsaturated" if it contains less. Examples of unsaturated carbon chains:
The hydrogens are required to stabilize the carbon chain.
If there is a double or triple bond then the electrons can be mobile and assume
different states. The molecule exists in a quantum-mechanical resonance
between the possible states.
Suppose we make a chain of atoms that is saturated in hydrogen. The following
table gives the longest stable chain for each element, and the longest stable
chain in an oxygen environment. Only carbon is capable of making long chains.
We can expect that aliens will be carbon-based.
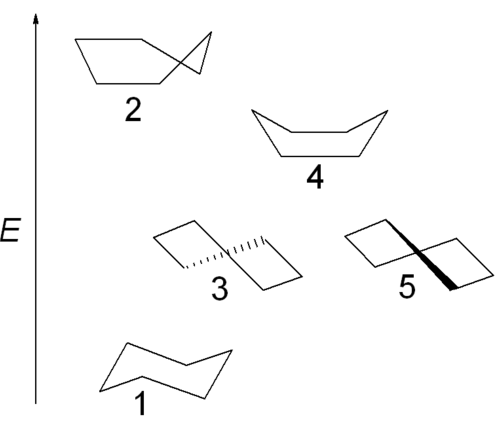
Cyclohexane comes in different conformations with different energies.
Benzine is a resonance molecule.
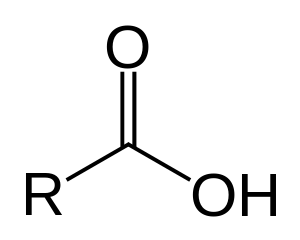
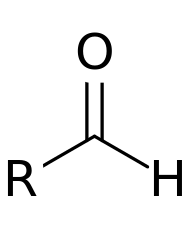
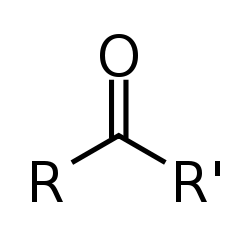
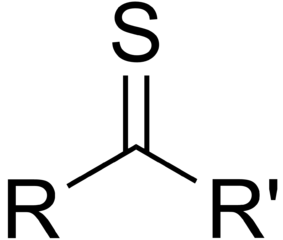
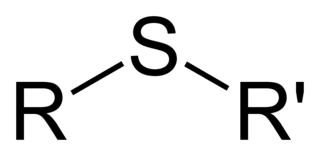
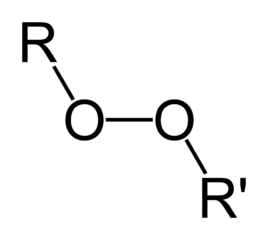
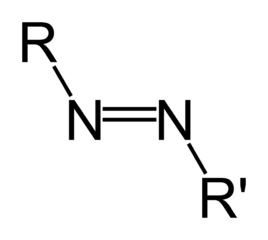
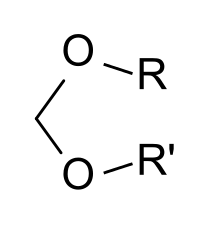
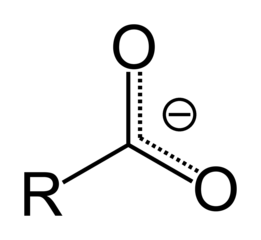
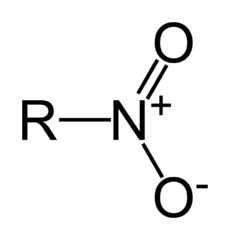
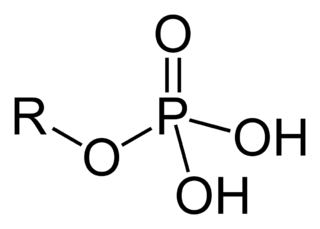
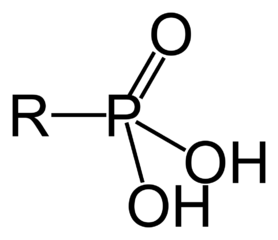
Organic molecules are classified by their functional group. "R" stands for
an arbitrary molecule.
Reacting hydrocarbons in an oxygen atmosphere yields the optimal power-to-weight ratio.
Given the enormous power required by brains, if intelligent life exists in the universe,
it likely gets its energy from reacting hydrocarbons in an oxygen atmosphere.
Most likely we would be able to eat their food.
Life appeared on the Earth within a billion years of its formation.
http://en.wikipedia.org/wiki/Timeline_of_evolutionary_history_of_life
Shortly after that, between 3500 and 3800 million years ago, the "Last
Universal Common Ancestor" lived.
http://en.wikipedia.org/wiki/Last_Universal_Common_Ancestor
The LUCA had the following properties:
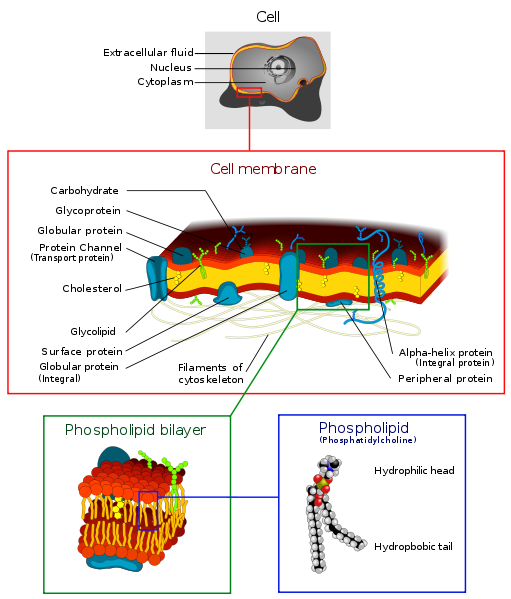
Single-cellular with a bilipid cell wall.
ATP to power enzymes.
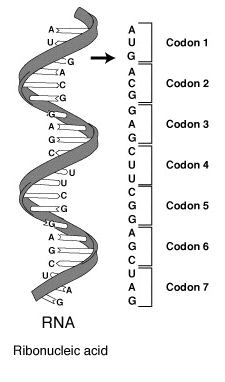
A DNA codon system with 4^3=64 options coding for 20 proteins.
This code hasn't changed since.
Cell walls are formed from a double layer of lipids. They are elastic and they
self-assemble.
Each lipid has a polar and a non-polar end. The polar end faces the water
and the non-polar end faces another lipid.
* Video of the self-assembly of a bilipid layer
If life were to exist in a non-polar solvent it would have to find another way
to make cell walls.
Enzymes use ATP as an energy source to power chemical reactions. ATP and ATP
synthase are common to all Earth life.
* Video of the ATP synthase enzyme in action
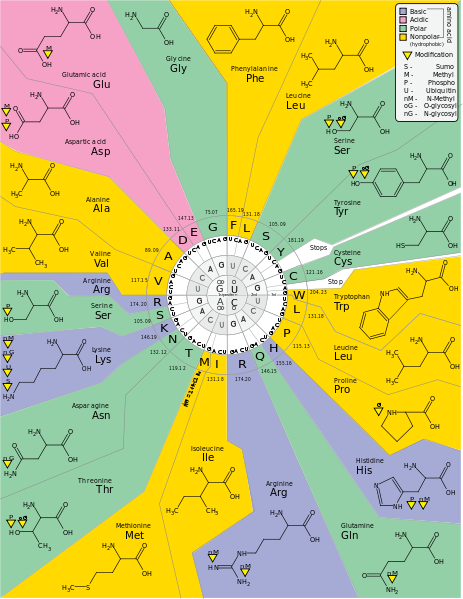
Amino acids have the above form, where R stands for an arbitrary molecule.
DNA codes a sequence of amino acids. The 64-element codon system is universal to
Earth life.
The codon ATG both codes for methionine and serves as an initiation site: the
first ATG in an mRNA's coding region is where translation into protein begins.
21 amino acids are used by eucaryote. More than 500 amino acids are known.
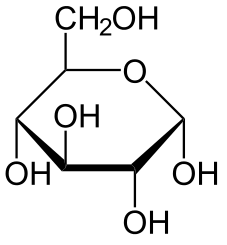
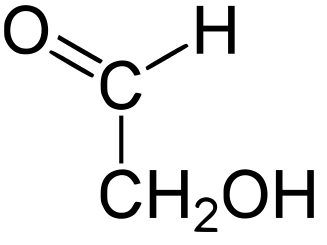
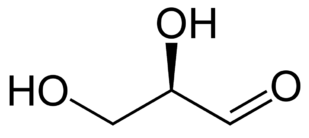
A sugar generally has the formula CN H2N ON, where
N = 2, 3, etc. The common sugars are hexoses with N=6.
Each sugar molecule has two mirror-symmetric forms, the "D" and "L" form. Only the D forms
are found in nature.
The following figures show all sugars up to 6 carbons. All can be metabolized by humans.
2 carbons:
3 carbons:
4 carbons:
5 carbons:
6 carbons:
Fatty acids and sugars are metabolized in the following stages, with each stage
yielding energy.
Blood delivers fatty acids to cells.
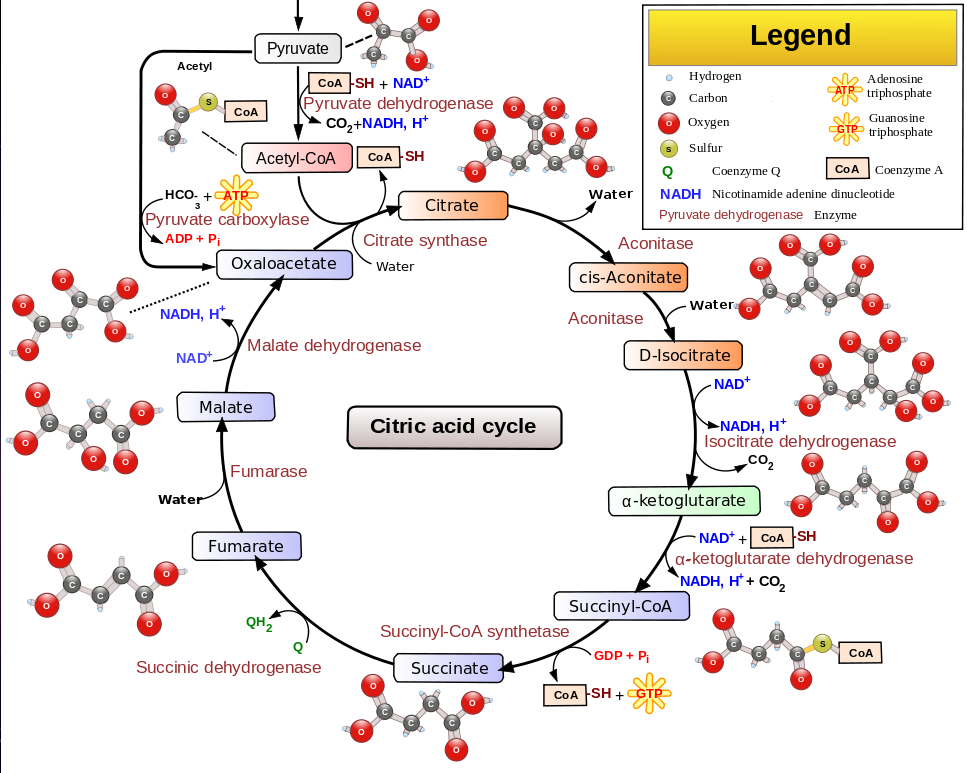
The citric acid cycle (Krebs cycle) converts acetyl or pyrovate into H2O and CO2.
Coenzyme-A carries the acetyl around.
A fat molecule is converted into a fatty acid by lipolysis, and then the fatty acid is converted
into acetyl by beta oxydation, and then the acetyl is converted into H2O and CO2
by the citric acid cycle.
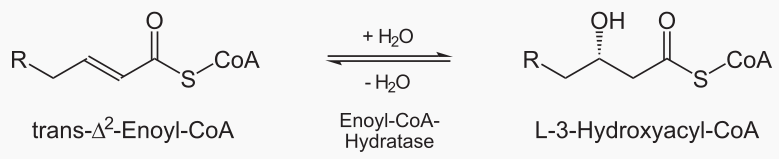
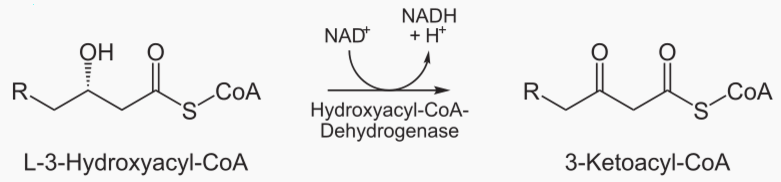
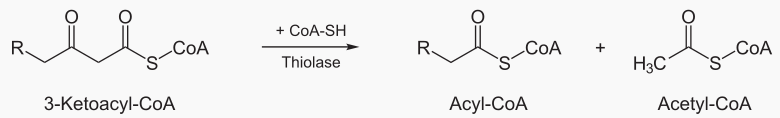
Beta oxidation cleaves 2 carbons from a fatty acid, which becomes acetyl. This process is repeated
until te entire fatty acid has been converted into acetyls.
The steps of beta oxidation are:
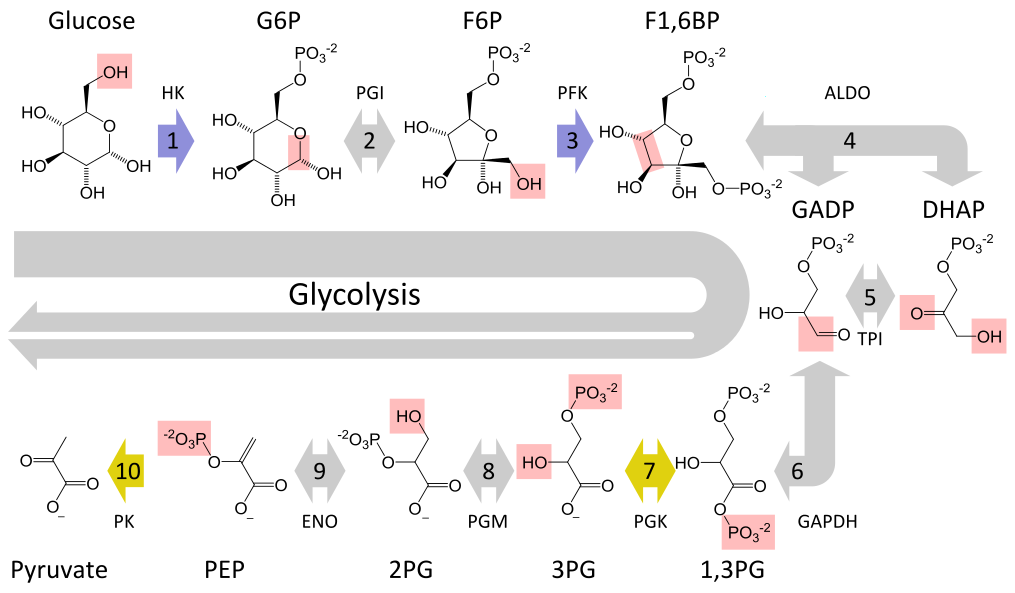
Glycolysis converts a glucose molecule into 2 pyrovate molecules. A summary of the reaction showing only
the starting and ending points is:
The full reaction is:
The citric acid cycle (Krebs cycle) converts acetyl or pyrovate into H2O and CO2.
Fat metabolism oxidizes a carbon chain so that the chain can be split into acetyl.
The strategy of the citric acid cycle is to further oxidize the acetyl (now a part of citrate)
so that the remaining carbon bonds in the acetyl can be broken.
An alcohol is a carbon chain with one OH attached.
Palmitic acid has 16 carbons and is the most common fatty acid found in food.
Weakly toxic:
Metals are held by a cofactor, which is held by a protein. Many cofactors
are porphyrin rings conposed of 4 pyrroles. Examples of porphyrins:
Oxygen bonds to the iron in a heme molecule and becomes superoxide.
All chlorophyll uses magnesium.
Zinc stabilizes the proteins that manipulate DNA and RNA.
Superoxide dimutase converts superoxide to oxygen or hydrogen peroxide.
The peroxidase enzyme decomposes hydrogen peroxide to water. Peroxidase contains
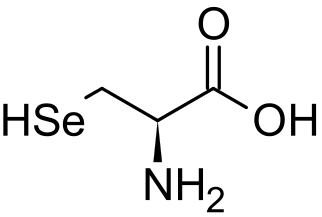
the selenocysteine amino acid, which contains selenium.
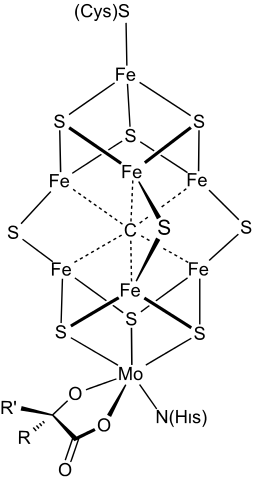
Nitrogen fixase uses an iron-molybdenum cofactor.
Selenium is a component of the amino acid selenocysteine.
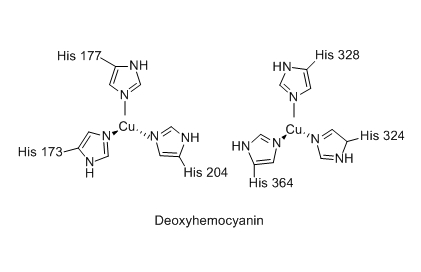
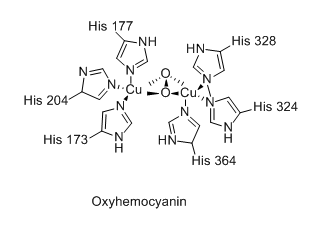
The hemocyanin protein uses copper to carry oxygen. It has an oxygen density that is
1/4 of hemoglobin.
Plastocyanin is a copper-containing protein used in photosynthesis.
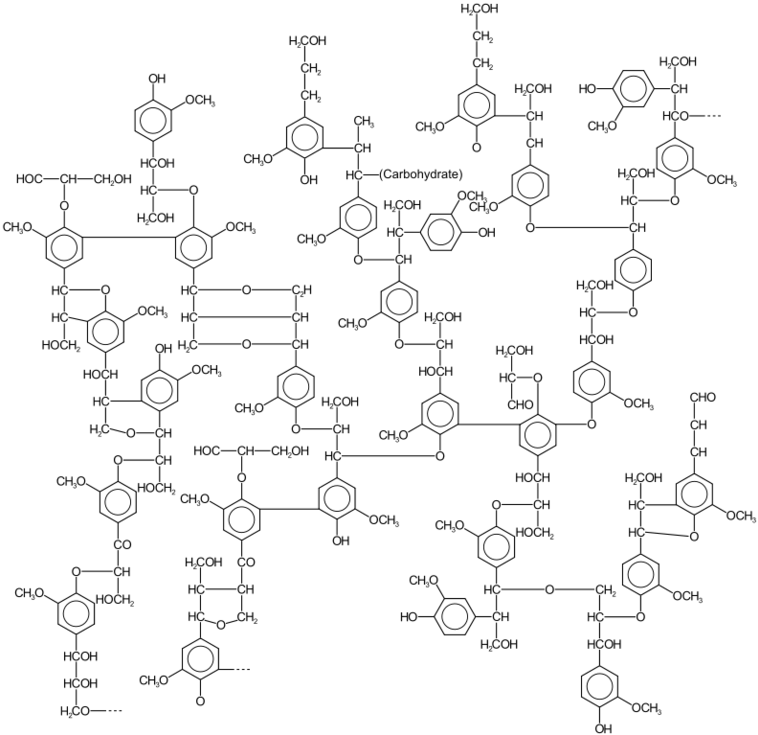
Lignin is the structural component of wood.
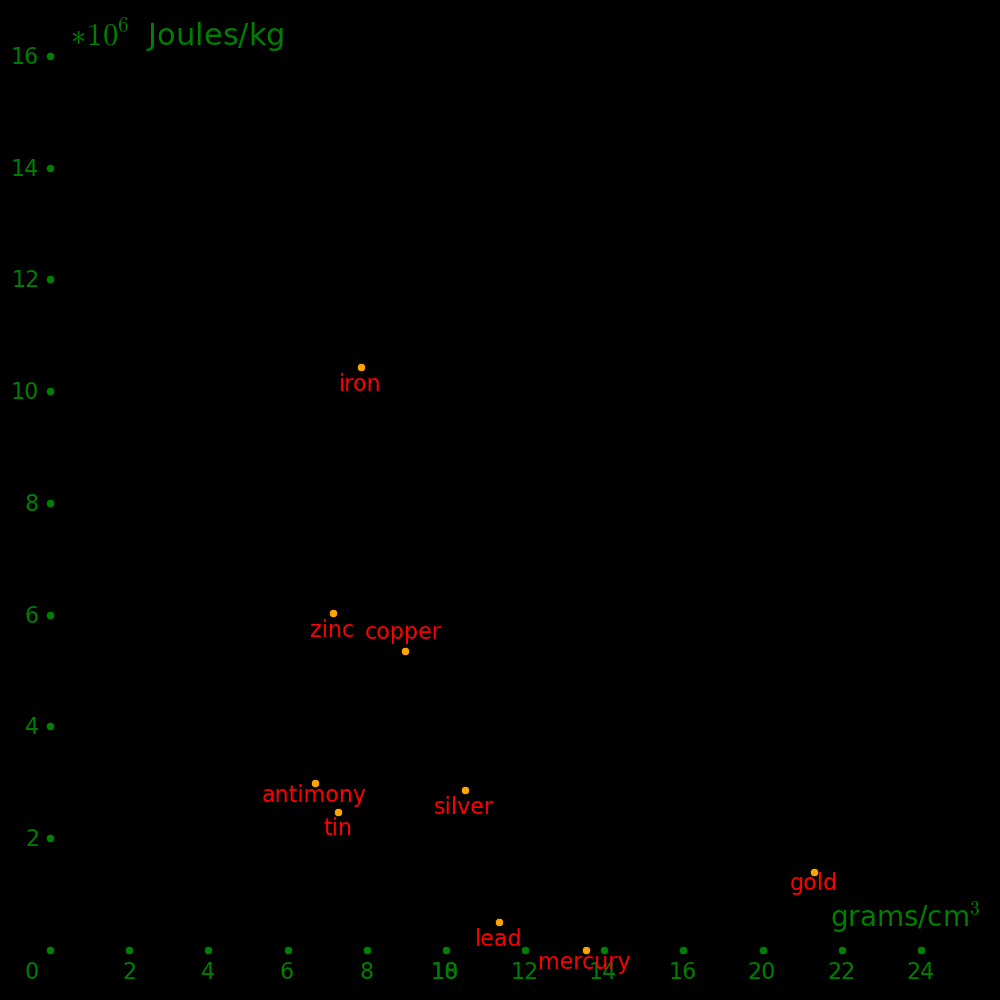
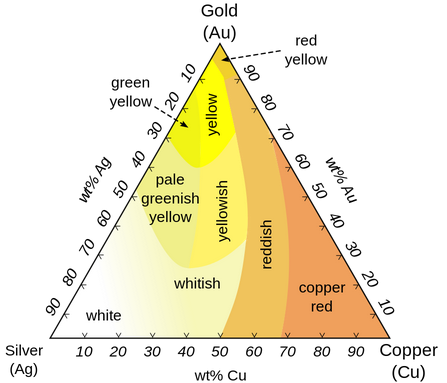
The earliest metals were gold and silver, the only ones that occur naturally in pure form.
Iron can occasionally be found as iron meteorites.
Copper was discovered around 7000 BCE by smelting copper minerals in a wood fire.
Around 3200 BCE it was found that copper is strenghened by tin, and this is called bronze.
Around 2000 BCE it was found that copper is also strengthed by zinc, and this is called brass.
The earliest metals were smeltable with a wood fire and they consist of copper, lead, silver, tin, zinc, and mercury.
They come from the following minerals:
Gold and silver were known since antiquity, but gold mining didn't start until 6000 BC, and silver smelting didn't
start until 4000 BC.
The minerals that were used by ancient civilizations to smelt metal are:
The next metal to be discovered was iron (c. 1200 BC), which requires a bellows-fed coal fire to smelt.
No new metals were discovered until cobalt in 1735. Once cobalt was discovered, it was realized that
new minerals may have new metals, and the race was on to find new minerals. This yielded
nickel, chromium, manganese, molybdenum, and tungsten.
Chromium is lighter and stronger than steel and it was discovered in 1797. It
satisfies the properties of "Valyrian steel" from Game of Thrones. There's no reason chromium couldn't have
been discovered earlier.
Coal smelting can't produce the metals lighter than chromium. For these you
need electrolysis. The battery was invented in 1799, enabling electrolysis, and
the lighter metals were discovered shortly after. These include aluminum, magnesium, titanium,
and beryllium.
Carbon fiber eclipses metals. The present age could be called the carbon age. The carbon age became
mature in 1987 when Jimmy Connors switched from a wood to a carbon racket.
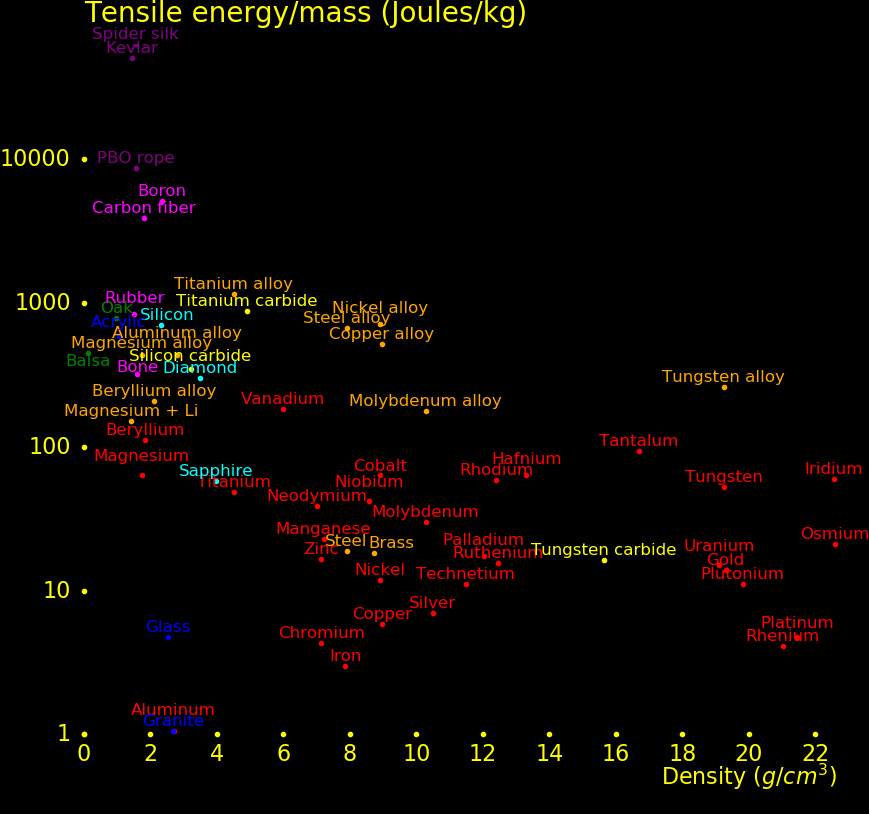
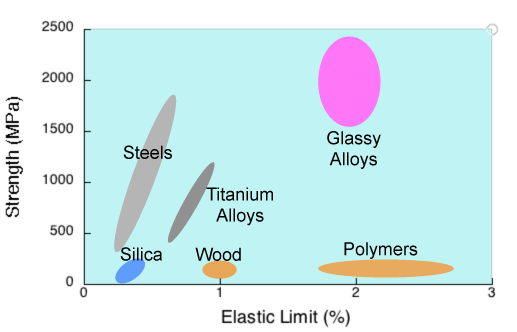
The plot shows the strength of materials.
Wood rivals alloys for strength.
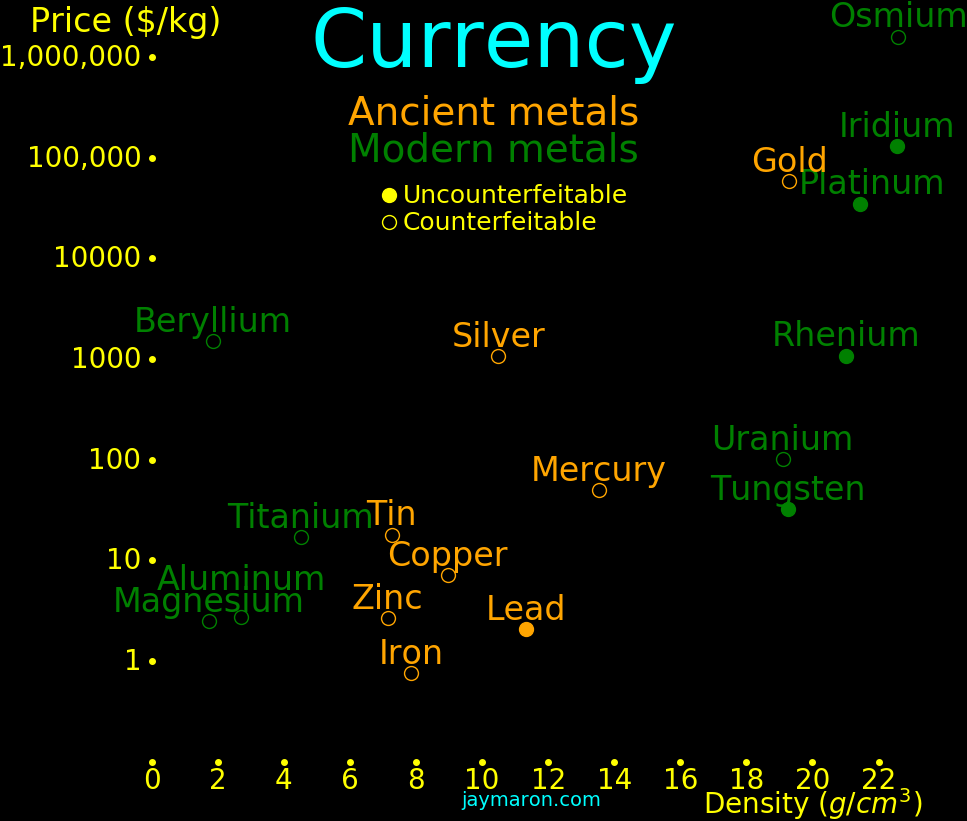
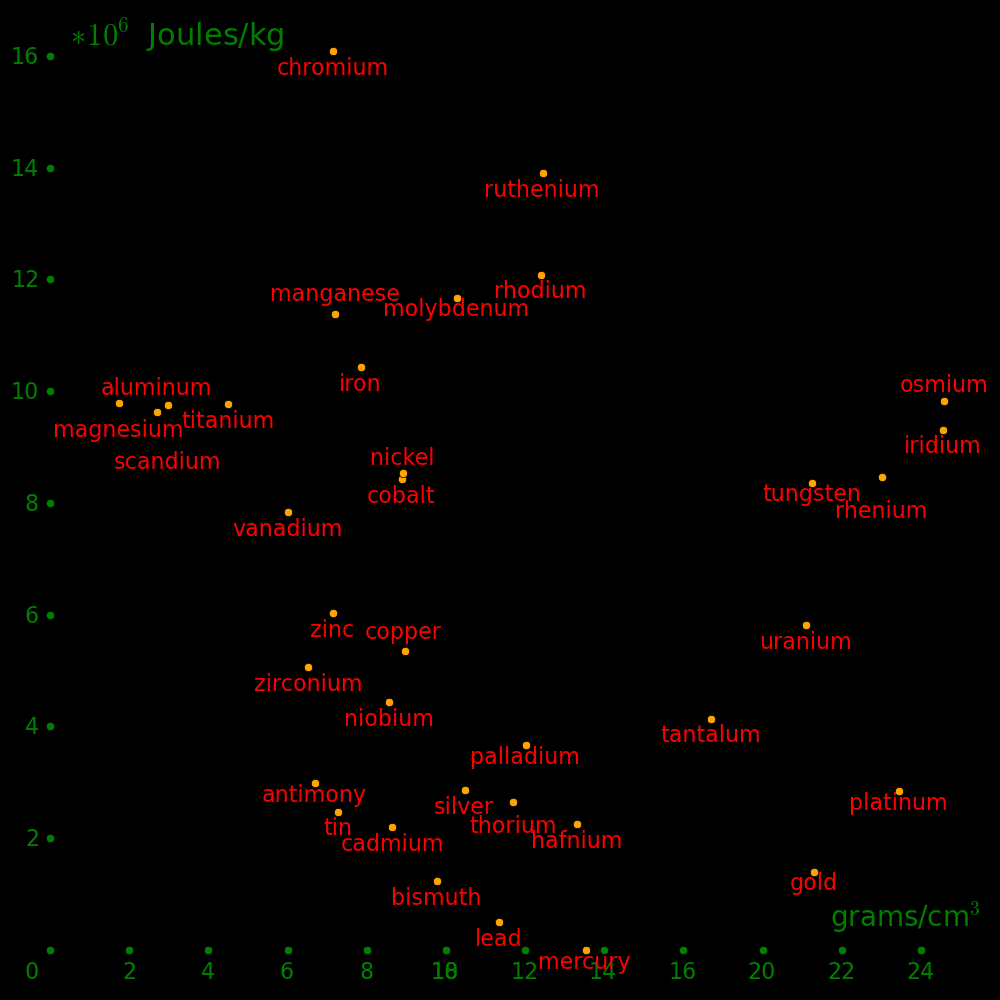
Gold was the densest element known until the discovery of platinun in 1735. It
was useful as an uncounterfeitable currency until the discovery of tungsten in
1783, which has the same density as gold. Today, we could use iridium,
platinum, or rhenium as an uncounterfeitable currency.
Prior to 1800, metals were obtained by smelting minerals, and the known metals
were gold, silver, copper, iron, tin, zinc, mercury, cobalt, manganese,
chromium, molybdenum, and tungsten. Elements to the left of chromium titanium
and scandium cant's be obtained by smelting, and neither can aluminum,
magnesium, and beryllium. They require electrolysis, which was enabled by
Volta's invention of the battery in 1799.
Prior to 1800, few elements were known in pure form. Electrolyis enabled the
isolation of most of the rest of the elements. The periodic table then became
obvious and was discovered by Mendeleev 1871. The battery launched modern
chemistry, and the battery could potentially have been invented much earlier.
Electrolysis enabled the isolation of sodium and potassium in 1807, and these were used
to smelt metals that can't be smelted with carbon.
For a metal, the stiffness is characterized by the "shear strength" and the
sword worthiness is characterized by the shear strength over the density
(the "strength to weight ratio"). For example for iron,
This plot includes all metals with a strength/density at least as large as lead,
plus mercury.
Beryllium is beyond the top of the plot.
In prehistoric times iron meteorites were the only source of metallic iron.
They consist of 90% iron and 10% nickel.
New alloys have been discovered that are stronger and ligher than diamond. These alloys have an
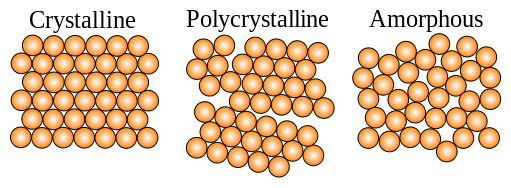
amorphous structure rather than the crystalline structure of conventional alloys.
A crystaline alloy tends to be weak at the boundaries between crystals and this limits
its strength. Amorphous alloys don't have these weaknesses and can be stronger.
Pure metals and alloys consisting of 2 or 3 different metals tend to be crystaline while alloys
with 5 or more metals tend to be amorphous. The new superalloys are mixes of at least 5 different
metals.
A material's strength is characterized by the "yield strength" and the quality is
the ratio of the yield strength to the density. This is often referred to as the "strength to weight ratio".
Most alloys weaken with increasing temperature except for a small subset called
"superalloys" that strengthen with temperature, such as Ni3Al and
Co3Al. This is called the "yield strength anomaly".
Nickel alloys in jet engines have a surface temperature of 1150 Celsius
and a bulk temperature of 980 Celsius. This is the limiting element for
jet engine performance. Half the mass of a jet engine is superalloy.
Current engines use Nickel superalloys and Cobalt superalloys are under
development that will perform even better.
Yield strength in GPa as a function of Celsius temperature.
Bells and cymbals are made from bell bronze, 4 parts copper and 1 part tin.
The "atomization energy" is the energy required to extract an atom
from an element in its raw form. For example,
The atomization energy of H2O is -971 kJ/mole.
Most metals are in oxidized form. The only metals that can be found in
pure form are gold, silver, copper, platinum, palladium, osmium, and iridium.
Smelting is a process for removing the oxygen to produce pure metal.
The ore is heated in a coal furnace and the carbon seizes the oxygen from
the metal. For copper,
For iron, the oxidation state is reduced in 3 stages until the
pure iron is left behind.
The following table gives the temperature required to smelt each element with
carbon.
The farther to the right on the periodic table, the lower the smelting
temperature, a consequence of "electronegativity".
The battery was invented in 1800, launching the field of electrochemistry
and enabling the the isolation of non-carbon-smeltable elements.
Davy used electrolysis in 1807 to isolate sodium and potassium and then he used
these metals to smelt other metals. To smelt beryllium with potassium,
BeO + 2 K ↔ Be + K2O.
Titanium can't be carbon smelted because it forms the carbide Ti3C.
For an expanded discussion of smelting physics, see jaymaron.com/metallurgy.html.
Thermite is smelting with aluminum. For example, to smelt iron with aluminum,
The following table shows reactions that change the oxidation state of a metal.
"M" stands for an arbitrary metal and the magnitudes are scaled to one mole of
O2. The last two columns give the oxidation state of the metal on
the left and right side of the reaction. An oxidation state of "0" is the pure
metal and "M2O" has an oxidation state of "1".
These elements are not necessarily on the Science Olympiad list.
We list minerals by element, with the most abundant mineral for each element listed first.
The composition of a typical opium poppy is:
High explosives have a large shock velocity.
The best oxidizer is liquid oxygen, and the exhaust speed for various fuels when
burned with oxygen is:
We use kerosene as a standard fuel and show the rocket speed for various oxidizers.
Some of the oxidizers can be used by themselves as monopropellants.
Above 550 Celsius, potassium nitrate decomposes. 2 KNO3 ↔ 2 KNO2 + O2.
The safety match was invented in 1844 by Pasch. The match head cannot ignite by
itself. Ignitition is achieved by striking it on a rough surface that contains
red phosphorus. When the match is struck, potassium chlorate in the match head
mixes with red phosphorus in the abrasive to produce a mixture that is easily ignited
by friction. Antimony trisulfide is added to increase the burn rate.
Before the invention of iron, fires were started by striking flint (quartz)
with pyrite to generate sparks. Flintlock rifles work by striking
flint with iron. With the discovery of cerium, ferrocerium replaced iron
and modern butane lighters use ferrocerium, which is still referred to as "flint".
Nitrous oxide is stored as a cryogenic liquid and injected along with gaoline
into the combustion chamber. Upon heating to 300 Celsius the nitrous oxide
decomposes into nitrogen and oxygen gas and releases energy.
The oxygen fraction in this gas is higher than that in air (1/3 vs. .21) and the
higher faction allows for more fuel to be consumed per cylinder firing.
Hydroquinone and peroxide are stored in 2 separate compartments are pumped into
the reaction chamber where they explode with the help of protein catalysts.
The explosion vaporizes 1/5 of the liquid and expels the rest as a boiling drop
of water, and the p-quinone in the liquid damages the foe's eyes. The energy
of expulsion pumps new material into the reaction chamber and the process
repeats at a rate of 500 pulses per second and a total of 70 pulses. The
beetle has enough ammunition for 20 barrages.
A turbojet engine compresses air before burning it to increase the flame speed
and make it burn explosively. A ramjet engine moving supersonically doesn't need
a turbine to achieve compression.
If the pressure front moves supersonically then the front forms a discontinuous
shock, where the pressure makes a sudden jump as the shock passes.
Metal powder is often included with explosives.
An oxygen candle is a mixture of sodium chlorate and iron powder,
which when ignited smolders at 600 Celsius and produces oxygen at a rate
of 6.5 man-hours of oxygen per kilogram of mixture. Thermal decomposition releases
the oxygen and the burning iron provides the heat. The products of the reaction are
NaCl and iron oxide.
The energy distribution for a 7.62 mm Hawk bullet is
The muzzle break at the end of the barrel deflects gas sideways to reduce recoil.
Corundum is a crystalline form of aluminium oxide (Al2O3). It is transparent in
its pure form and can have different colors when metal impurities are
present.
Lignin comprises 30 percent of wood and it is the principal structural element.
A string ideally has both large strength and large strain, which favors
Vectran.
Suppose Batman has a rope made out of Zylon, the strongest known polymer.
Valyrian steel is a fictional substance from "Game of Thrones" that is
stronger, lighter, and harder than steel. The only elements that qualify are
beryllium, titanium, and vanadium, none of which were known in Earth history
until the 18th century. Valyrian steel could be of these elements,
an alloy, or a magical substance. According to George Martin, magic is
involved.
The fact that it is less dense than steel means that it can't be a fancy form
of steel such as Damascus steel or Wootz steel. Also, fancy steel loses its
special properties if melted and hence cannot be reforged, whereas Valyrian steel
swords can be reforged.
In Earth history, the first metal discovered since iron was cobalt in 1735.
This launched a frenzy to smelt all known minerals and most of the smeltable
metals were discovered by 1800. Then the battery and electrochemstry were
discovered in 1800 and these were used to obtain the unsmeltable metals, which
are lithium, beryllium, magnesium, aluminum, titanium, vanadium, niobium, and
Uranium. Almost all of the strong alloys use these metals, and so the Valyrians
must have used either electrochemistry or magic to make Valyrian steel.
The following metals and alloys are both stronger and lighter than steel and
could hypothetically be Valyrian steel.
Petyr Baelish: Nothing holds an edge like Valyrian steel.
Tyrion Lannister: Valyrian steel blades were scarce and costly, yet thousands
remained in the world, perhaps two hundred in the Seven Kingdoms alone.
George Martin: Valyrian steel is a fantasy metal. Which means it has magical
characteristics, and magic plays a role in its forging.
George Martin: Valyrian steel was always costly, but it became considerably
more so when there was no more Valyria, and the secret of its making were lost.
Ned Stark's stord "Ice" is melted down and reforged into two smaller swords,
"Oathkeeper" and "Widow's Wail". This rules out Valyrian steel being Wootz steel
because Wootz steel loses its special properties when reforged.
Appearances of Valyrian steel in Game of Thrones:
The burn rate of gasoline is limited by the supply of oxygen.
Copper burns with a green flame. Adding copper powder to the explosive adds
energy to the blast.
Three types of incendiaries are:
The reason air heats when compressed is because it is composed of atoms. You
can see this in action with the "Gas" simulation at phet.colorado.edu. You can
also see how atoms in a gas can carry a sound wave, and why the sound speed
has the same order-of-magnitude as the thermal velocity of the atoms.


1 2 3 4 3 2 1 0
Hydrogen Helium
Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon
Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
Potassium Calcium Gallium Germanium Arsenic Selenium Bromine Krypton
Lithium 1 Potassium 1
Beryllium 2 Calcium 2
Scandium 3
Sodium 1 Titanium 4
Magnesium 2 Vanadium 4
Aluminum 3 Chromium 4
Manganese 4
Iron 3
Cobalt 2
Nickel 2
Copper 3
Zinc 2


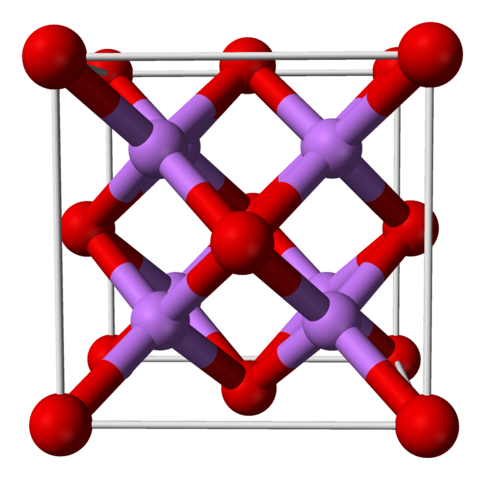
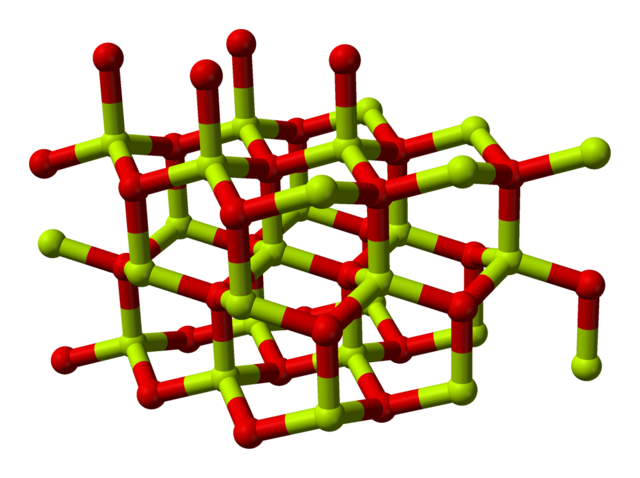

1 electron: Cu2O Copper(I) Oxide. Cuprous oxide. Red paint
2 electrons: CuO Copper(II) Oxide. Cupric oxide. Black color
3 electrons: Cu2O3 Copper(III) Oxide
"Electrons" refers to the number of electrons that each copper atom gives to the
oxygen atoms.
3 electons: Fe2O3 Iron(III) Oxide. Ferric oxide. Most common form
2 electrons: FeO Iron(II) oxide. Rare
2 or 3 electrons: Fe3O4 Iron(II,III) Oxide. Magnetite
e-
1 H 1 H2O Water
2 He 0 Does not react with oxygen
3 Li 1 Li2O
4 Be 2 BeO Beryllium oxide
5 B 3 B2O3 Boron trioxide. Most common form. High conductivity
1/3 B6O Boron suboxide. High conductivity and hardness
6 C 4 CO2 Carbon dioxide
2 CO Carbon monoxide. Toxic. Displaces oxygen from hemoglobin
7 N 1 N2O Nitrous oxide. Laughing gas
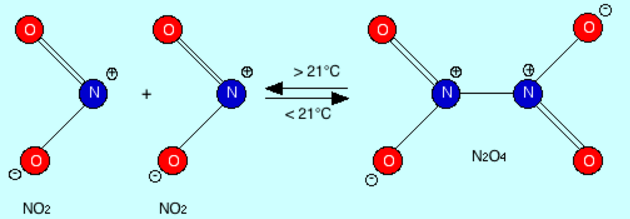
2 NO Nitric oxide. Gas. Signaling molecule. Decomposes in air to NO2
4 NO2 Nitrogen dioxide. Toxic gas
4 N2O4 Dinitrogen tetroxide
5 N2O5 Dinitrogen pentoxide
8 O O2 Oxygen
O3 Ozone
9 F -2 OF2 Oxygen difluoride
10 Ne 0 Does not react with oxygen
11 Na 1 Na2O Sodium oxide
12 Mg 2 MgO Magnesium oxide
13 Al 3 Al2O3 Aluminum(III) oxide. Aluminum oxide. Most common form
2 AlO Aluminum(II) oxide. Aluminum monoxide
1 Al2O Aluminum(I) oxide
14 Si 4 SiO2 Quartz
15 P 4 P2O4 Diphosphorus tetroxide
3 P4O6 Phosphorus trioxide. Phosphorus(III) oxide. Stable. Reacts with water
5 P4O10 Phosphorus pentoxide
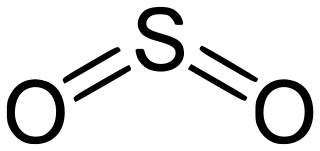
16 S 6 SO3 Sulfur trioxide. Component of acid rain
4 SO2 Sulfur dioxide. Toxic gas
2 SO Sulfur monoxide. Unstable
17 Cl 4 ClO2 chlorine dioxide
2 ClO Foe of the ozone layer
1 Cl2O Dichlorine monoxide. Unstable. Explosive
18 Ar 0 Does not react with oxygen
19 K 1 K2O Potassium oxide
20 Ca 2 CaO Calcium oxide Quicklime
21 Sc 3 Sc2O3 Scandium(III) oxide. Ceramic
22 Ti 4 TiO2 Titanium dioxide. Most common form
3 Ti2O3 Dititanium trioxide
2 TiO Titanium monoxide. Corundum structure. Tistarite ore (extremely rare)
23 V 5 V2O5 Vanadium(V) oxide. Rare mineral
4 VO2 Vanadium(IV) oxide
3 V2O3 Vanadium(III) oxide. Morphs in air to V2O4
2 VO Vanadium(II) oxide
24 Cr 2 CrO Chromium(II) oxide
3 Cr2O3 Chromium(III) oxide. Eskolaite ore
4 CrO2 Chromium dioxide
6 CrO3 Chromium trioxide
25 Mn 7 Mn2O7 Manganese(VII) oxide. Extremely unstable
6 MnO3 Manganese(VI) oxide
4 MnO2 Manganese dioxide. Most common form
3 Mn2O3 Manganese(III) oxide
8/3 Mn3O4 Manganese(II,III) oxide
2 MnO Manganese(II) oxide. Rare mineral
26 Fe 3 Fe2O3 Iron(III) Oxide. Ferric oxide. Most common form
2 FeO Iron(II) oxide. Rare
8/3 Fe3O4 Iron(II,III) Oxide. Magnetite
27 Co 3 Co2O3 Cobalt(II) oxide. Cobaltic oxide
2 CoO Cobalt(II) oxide. Cobaltous oxide
8/3 Co3O4 Cobalt(II,IIIs) oxide. Cobaltous oxide
28 Ni 2 NiO Nickel(II) oxide
29 Cu 1 Cu2O Copper(I) Oxide. Cuprous oxide. Red paint
2 CuO Copper(II) Oxide. Cupric oxide. Black color. Common ore
3 Cu2O3 Copper(III) Oxide
30 Zn 2 ZnO Zinc oxide
30 Ga 1 GaO2 Gallium(I) oxide
Ga 3 Ga2O3 Gallium(III) oxide
32 Ge 2 GeO2 Germanum oxide
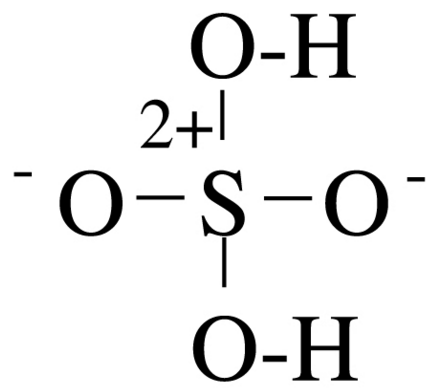
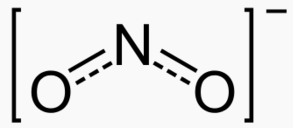
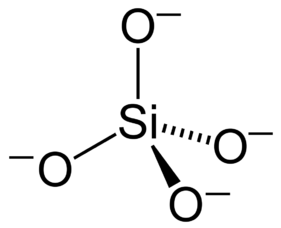
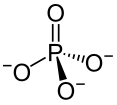
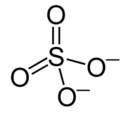
Type Example
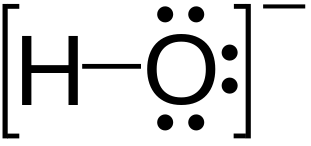
Hydroxide HOH Hydrogen hydroxide
Hypofluorite HFO Hypoflourous acid
Hypochlorite HClO Hypochlorous acid
Peroxide H2O2 Hydrogen peroxide
Carbide WC Tungsten carbide
Oxide H2O Hydrogen oxide
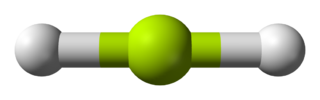
Fluoride HF Hydrogen fluoride
Silicide H4Si Hydrogen silicide
Phosphide H3P Hydrogen phosphide
Sulfide H2S Hydrogen sulfide
Chloride HCl Hydrogen chloride
Arsenide H3As Hydrogen arsenide
Selenide H2Se Hydrogen selenide
Bromide HBr Hydrogen bromide
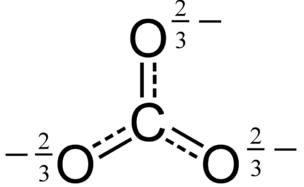
Carbonate H2CO3 Carbonic acid
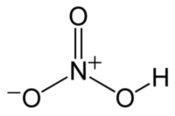
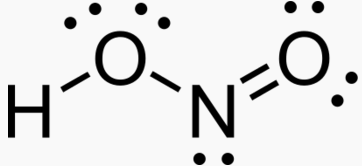
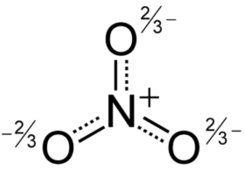
Nitrate HNO3 Nitric acid
Aluminate H5AlO4 Hydrogen aluminate
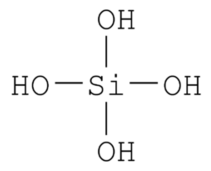
Silicate H4SiO4 Silicic acid
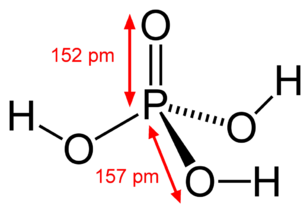
Phosphate H3PO4 Phosphoric acid
Sulfate H2SO4 Sulfuric acid
Chlorate HClO3 Hydrogen chlorate
Perchlorate HClO4 Hydrogen perchlorate
Germanate H4GeO4 Hydrogen germanate
Arsenate H3AsO4 Arsenic acid
Selenate H2SeO4 Hydrogen selenate
Bromate HBrO3 Hydrogen bromate
Tellurate H2TeO4 Hydrogen tellurate
Iodate HIO3 Hydrogen iodate
Nitrite HNO2 Nitrous acid
Chlorite HClO2 Hydrogen chlorite

![]()
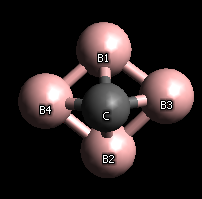
Carbon atoms per metal atom
Boron 1/4
Silicon 1
Titanium 1
Beryllium 1/2
Zirconium 1/2
Tantalum 1
Tungsten 1
Aluminum 3/4

Element Molecule Bond energy
(eV)
H H2 4.52
Li LiH 2.56
Be BeH2 2.35
B BH3 3.43
C CH4 3.52
N NH3 3.26
O H2O 4.41
F HF 4.90
Na NaH 2.09
Mg MgH2 2.04
Al AlH3 2.96
Si SiH4 3.10
P PH3 3.56
S H2S 3.57
Cl HCl 4.48
K KH 1.90
Ca CaH2 1.74
Ga ?
Ge 3.36
As 2.82
Se SeH2 3.17
Br HBr 3.80
Pressure = P (Pascals or Newtons/meter2 or Joules/meter3)
Temperature = T (Kelvin)
Volume = Vol (meters3)
Total gas kinetic energy = E (Joules)
Kinetic energy per volume = e = E/Vol (Joules/meter3)
Number of gas molecules = N
Mass of a gas molecule = M
Gas molecules per volume = n = N / Vol
Gas density = D = N M / Vol
Avogadro number = Avo= 6.022⋅1023 moles-1
Moles of gas molecules = Mol= N / Avo
Boltzmann constant = k = 1.38⋅10-23 Joules/Kelvin
Gas constant = R = k Avo = 8.31 Joules/Kelvin/mole
Gas molecule thermal speed = Vth
Mean kinetic energy / gas molecule= ε = E / n = ½ M Vth2 (Definition of the mean thermal speed)
Gas pressure arises from the kinetic energy of gas molecules and has units of
energy/volume.
The ideal gas law can be written in the following forms:
P = 2⁄3 e Form used in physics
= R Mol T / Vol Form used in chemistry
= k N T / Vol
= 1⁄3 N M Vth2/ Vol
= 1⁄3 D Vth2
= k T D / M
Gas simulation at phet.colorado.edu
Derivation of the ideal gas law
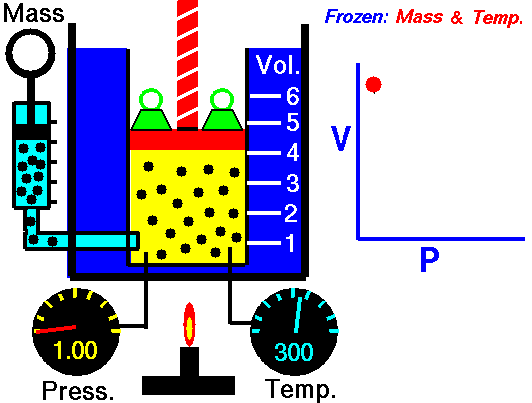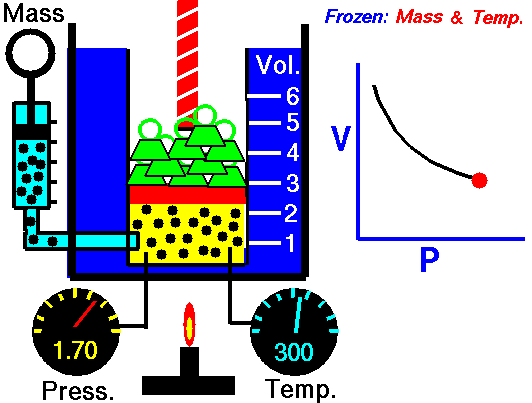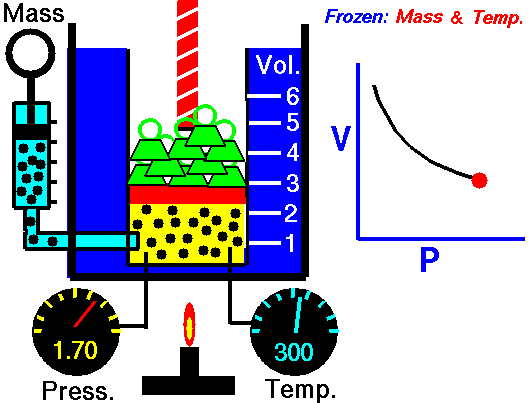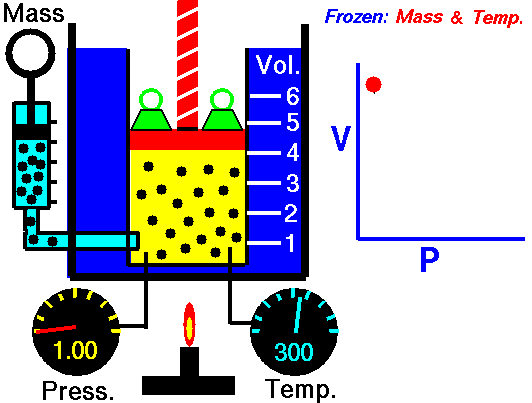
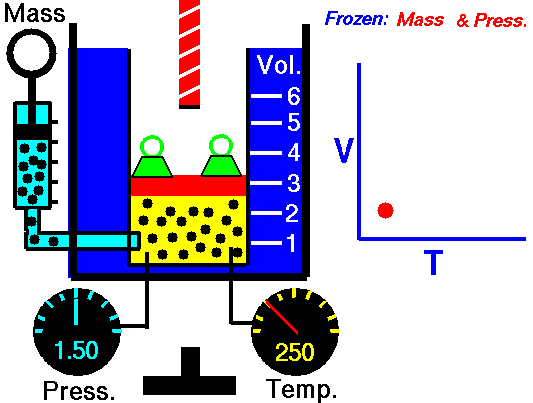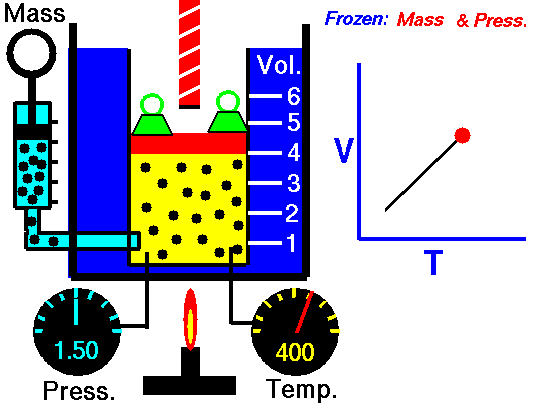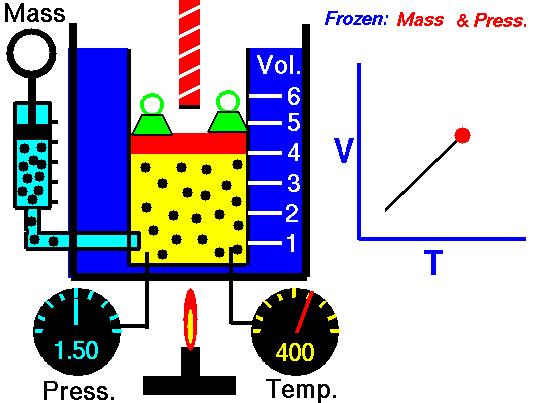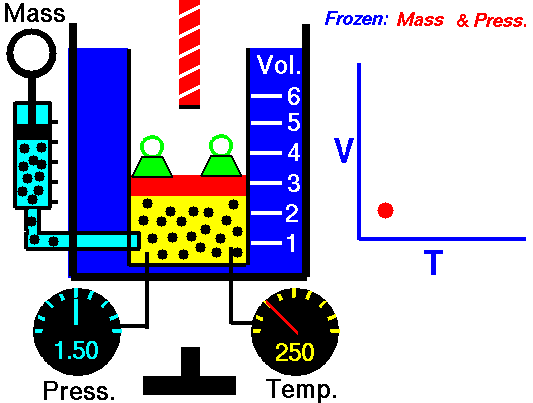
1660 Boyle law P Vol = Constant at fixed T
1802 Charles law T Vol = Constant at fixed P
1802 Gay-Lussac law T P = Constant at fixed Vol
1811 Avogadro law Vol / N = Constant at fixed T and P
1834 Clapeyron law P Vol / T = Constant combined ideal gas law
Molecule mass = M
Thermal speed = Vth
Boltzmann constant = k = 1.38⋅10-23 Joules/Kelvin
Molecule mean kinetic energy = ε
A gas molecule moving in N dimensions has N degrees
of freedom. In 3D the mean energy of a gas molecule is
ε = 3⁄2 k T = ½ M V2th
Adiabatic constant = γ
= 5/3 for monatomic molecules such as helium, neon, krypton, argon, and xenon
= 7/5 for diatomic molecules such as H2, O2, and N2
= 7/5 for air, which is 21% O2, 78% N2, and 1% Ar
≈ 1.31 for a triatomic gas such as CO2
Pressure = P
Density = D
Sound speed = Vsound
Mean thermal speed = Vth
K.E. per molecule = ε = ½ M Vth2
V2sound = γ P / D = 1⁄3 γ V2th
The sound speed depends on temperature and not on density or pressure.
Vsound = .68 Vth
These laws are derived in the appendix.
M in atomic mass units
Helium atom 4
Neon atom 20
Nitrogen molecule 28
Oxygen molecule 32
Argon atom 40
Krypton atom 84
Xenon atom 131
A helium atom has a smaller mass than a nitrogen molecule and hence has a
higher sound speed. This is why the pitch of your voice increases if you
inhale helium. Inhaling xenon makes you sound like Darth Vader. Then you pass
out because Xenon is an anaesthetic.
1635 Gassendi measures the speed of sound to be 478 m/s with 25% error.
1660 Viviani and Borelli produce the first accurate measurement of the speed of
sound, giving a value of 350 m/s.
1660 Hooke's law published. The force on a spring is proportional to the change
in length.
1662 Boyle discovers that for air at fixed temperature,
Pressure * Volume = Constant
1687 Newton publishes the Principia Mathematica, which contains the first analytic
calculation of the speed of sound. The calculated value was 290 m/s.
Newton's calculation was correct if one assumes that a gas behaves like Boyle's
law and Hooke's law.
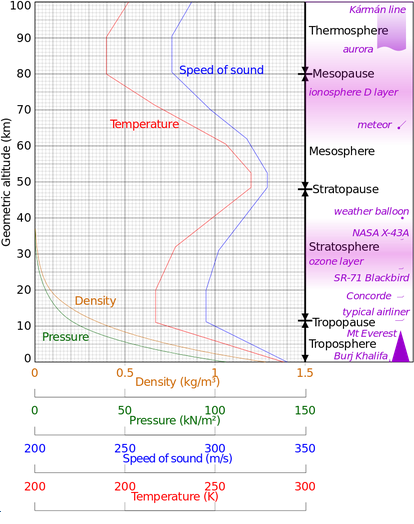
Melt Boil Solid Liquid Gas Mass Sound speed
(K) (K) density density density (AMU) at 20 C
g/cm3 g/cm3 g/cm3 (m/s)
He .95 4.2 .125 .000179 4.00 1007
Ne 24.6 27.1 1.21 .000900 20.18
Ar 83.8 87.3 1.40 .00178 39.95 319
Kr 115.8 119.9 2.41 .00375 83.80 221
Xe 161.4 165.1 2.94 .00589 131.29 178
H2 14 20 .070 .000090 2.02 1270
N2 63 77 .81 .00125 28.01 349
O2 54 90 1.14 .00143 32.00 326
Air .0013 29.2 344 79% N2, 21% O2, 1% Ar
H2O 273 373 .917 1.00 .00080 18.02
CO2 n/a 195 1.56 n/a .00198 44.00 267
CH4 91 112 .42 .00070 16.04 446
CH5OH 159 352 .79 .00152 34.07 Alcohol
Gas density is for 0 Celsius and 1 Bar. Liquid density is for the boiling point,
except for water, which is for 4 Celsius.
M = Mass of a gas molecule
V = Thermal speed
E = Mean energy of a gas molecule
= 1/2 M V^2
H = Characteristic height of an atmosphere
g = Gravitational acceleration
Suppose a molecule at the surface of the Earth is moving upward with speed V and suppose
it doesn't collide with other air molecules. It will reach a height of
M H g = 1/2 M V^2
This height H is the characteristic height of an atmosphere.
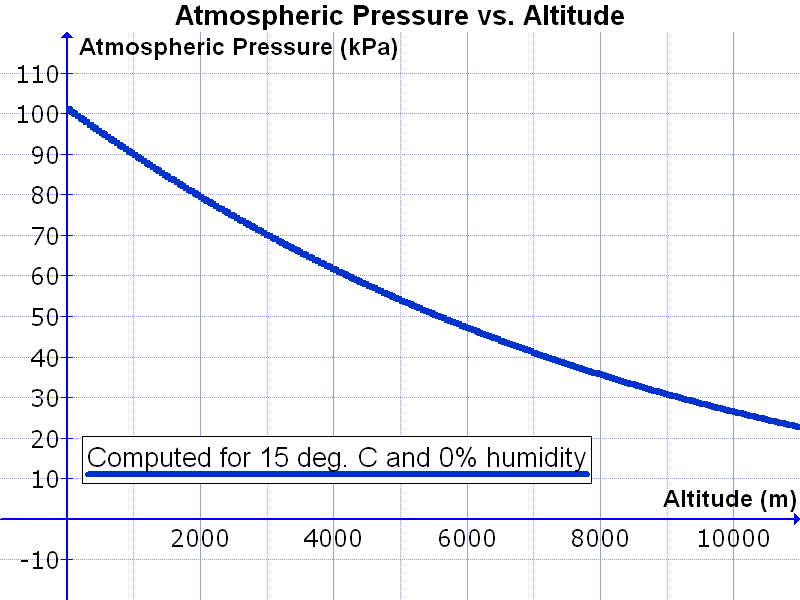
Pressure of air at sea level = 1 Bar
Pressure of air in Denver = .85 Bar One mile high
Pressure of air at Mount Everest = 1/4 Bar 10 km high
The density of the atmosphere scales as
Density ~ (Density At Sea Level) * exp(-E/E0)
where E is the gravitational potential energy of a gas molecule and E0 is the
characteristic thermal energy given by
E0 = M H g = 1/2 M V^2
Expressed in terms of altitude h,
Density ~ Density At Sea Level * exp(-h/H)
For oxygen,
E0 = 3/2 * Boltzmann_Constant * Temperature
E0 is the same for all molecules regardless of mass, and H depends on the
molecule's mass. H scales as
H ~ Mass^-1
S = Escape speed
T = Temperature
B = Boltzmann constant
= 1.38e-23 Joules/Kelvin
g = Planet gravity at the surface
M = Mass of heavy molecule m = Mass of light molecule
V = Thermal speed of heavy molecule v = Thermal speed of light molecule
E = Mean energy of heavy molecule e = Mean energy of light molecule
H = Characteristic height of heavy molecule h = Characteristic height of light molecule
= E / (M g) = e / (m g)
Z = Energy of heavy molecule / escape energy z = Energy of light molecule / escape energy
= .5 M V^2 / .5 M S^2 = .5 m v^2 / .5 m S^2
= V^2 / S^2 = v^2 / S^2
For an ideal gas, all molecules have the same mean kinetic energy.
E = e = 1.5 B T
.5 M V^2 = .5 m v^2 = 1.5 B T
The light molecules tend to move faster than the heavy ones. This is why
your voice increases in pitch when you breathe helium. Breathing a heavy gas such
as Xenon makes you sound like Darth Vader.
V^2 << S^2 <-> Z << 1
Escape Atmos Temp H2 N2 Z Z
speed density (K) km/s km/s (H2) (N2)
km/s (kg/m^3)
Jupiter 59.5 112 1.18 .45 .00039 .000056
Saturn 35.5 84 1.02 .39 .00083 .00012
Neptune 23.5 55 .83 .31 .0012 .00018
Uranus 21.3 53 .81 .31 .0014 .00021
Earth 11.2 1.2 287 1.89 .71 .028 .0041
Venus 10.4 67 735 3.02 1.14 .084 .012
Mars 5.03 .020 210 1.61 .61 .103 .015
Titan 2.64 5.3 94 1.08 .41 .167 .024
Europa 2.02 0 102 1.12 .42 .31 .044
Moon 2.38 0 390 2.20 .83 .85 .12
Ceres .51 0 168 1.44 .55 8.0 1.14
Even if an object has enough gravity to capture an atmosphere, it can still lose it
to the solar wind. Also, the upper atmosphere tends to be hotter than at the
surface, increasing the loss rate.
Thermal Speed < 1/5 Escape speed
Thermal speed of molecules ~ Escape speed
In the gas simulation at phet.colorado.edu, you can move the wall and watch the
gas change temperature.
3 * Boltzmann_Constant * Temperature ~ MassOfMolecules * Escape_Speed^2
For the sun, what is the temperature of a proton moving at the escape speed?
This sets the scale of the temperature of the core of the sun. The minimum temperature
for hydrogen fusion is 4 million Kelvin.
Escape speed (km/s) Core composition
Sun 618. Protons, electrons, helium
Earth 11.2 Iron
Mars 5.03 Iron
Moon 2.38 Iron
Ceres .51 Iron
M = Mass of molecule
V = Speed of the molecule
L = Space between the walls
With each collision, the momentum change = 2 M V
Force = Change in momentum / Time between collisions = M V^2 / L
Suppose a gas molecule is in a cube of volume L^3 and a molecule bounces back
and forth between two opposite walls (never touching the other four walls).
The pressure on these walls is
Pressure = Force / Area
= M V^2 / L^3
= M V^2 / Volume
Pressure * Volume = M V^2
This is the ideal gas law in one dimension. For a molecule moving in 3D,
Velocity^2 = (Velocity in X direction)^2
+ (Velocity in Y direction)^2
+ (Velocity in Z direction)^2
Characteristic thermal speed in 3D = 3 * Characteristic thermal speed in 1D.
To produce the 3D ideal gas law, replace V^2 with 1/3 V^2 in the 1D equation.
Pressure * Volume = 1/3 M V^2 Where V is the characteristic thermal speed of the gas
This is the pressure for a gas with one molecule. If there are n molecules,
Pressure Volume = n 1/3 M V^2 Ideal gas law in 3D
If a gas consists of molecules with a mix of speeds, the thermal speed is defined as
Kinetic dnergy density of gas molecules = E = (n / Volume) 1/2 M V^2
Using this, the ideal gas law can be written as
Pressure = 2/3 E
= 1/3 Density V^2
= 8.3 Moles Temperature / Volume
The last form comes from the law of thermodynamics:
M V^2 = 3 B T
Total gravitational energy = -2 * Total kinetic energy
just like for a single satellite on a circular orbit.
Gravitational energy of the sun = -2 * Kinetic energy of protons in the sun
Mole of carbon-12 = 12 grams (exact)
Mole of hydrogen = 1.008 grams = 1.674⋅10-27 kg
Mole of water = 18.02 grams = 2.992⋅10-26 kg
Avogadro number = NAvo = 6.022⋅1023 molecules/moles-1
Water molecule density = NH2O = 3.343⋅1028 molecules/meter3
Molecule concentration = C (moles/Litre)
Water concentration = CH2O = 55.49 moles/Litre
H+ concentration = CH+ = 10-7 moles/Litre for pure water
OH- concentration = COH- = 10-7 moles/Litre for pure water
Acid concentration = Cx
Acid ion concentration = Cx-
Acid molecule mass = Mx
Water molecule mass = MH2O
Water mass density = ρH2O = 1000 g/Litre
Acid mass density = ρx = ρH2O (Mx/MH2O) (Cx/CH2O)
H2O dissociation rate = RH2O = 2.36⋅10-5 seconds-1
H+ + OH- association rate = ROH- = 1.3⋅1011 Litres/Mole/second
H2O dissociation time = TH2O = R-1H2O = 42370 seconds = 11.77 hours
Acid dissociation rate = Rx
H+ + X- association rate = Rx-
Water constant = KH2O = RH2O / ROH- (moles/Litre)
Acid constant = Kx = Rx / Rx-
Boltzmann constant = k = 1.381⋅10-23 Joules/Kelvin
Gibbs energy = E = - k NAvo T log10 K (Joules/mole)
Acid ionization fraction = Fx = Cx- / Cx
= (Kx/Cx-)½ if Cx ≪ Kx
≈ 1 if Cx ≫ Kx
Water dissociation: H+ + OH- → H2O Rate = RH2O CH2O moles/Litre/second
Water association: H2O → H+ + OH- Rate = ROH- CH+ COH- moles/Litre/second
For pure water in equilibrium, the dissociation and association rates are equal.
ROH- CH+ COH- = RH2O CH2O
CH2O = 55.49 moles/Litre
CH+ = COH-
C2H+ = RH2O / ROH- = KH2O = 10-14
For an acid,
Rx- CH+ Cx- = Rx Cx
CH+ Cx- / Cx = Kx = Rx / Rx-
If there is only one acid in the solution,
CH+ = Cx-
CH+ = (Kx Cx)½
The ionization fraction of the acid Cx-/Cx-
is
If Cx ≪ Kx then Cx-/Cx ≈ 1 the acid is fully ionized
If Cx ≫ Kx then Cx-/Cx = (Kx/Cx-)½ ≪ 1 the acid is partially ionized
K log10K Energy
(electron Volts)
Perchoric HClO4 131.8 2.1 -.125
Nitric HNO3 27.5 1.4 -.083
Hydronium H3O+ 1 0 0
Selenic H2SeO4 Large
Sulfuric H2SO4 Large
Chloric HCL Large
Chromic H2CrO4 1.8e-1 -.74 .0439
Iodic HIO3 1.7e-1 -.78 .0463
Phosphorous H3PO3 5.0e-2 -1.3 .077
Selenic HSeO4- 2.2e-2 -1.66 .098
Sulfurous H2SO3 1.4e-2 -1.85 .110
Chlorous HClO2 1.1e-2 -1.94 .115
Sulfuric HSO4- 1.0e-2 -1.99 .118
Phosphoric H3PO4 6.9e-3 -2.14 .127
Fluoric HF 6.3e-4 -3.20 .190
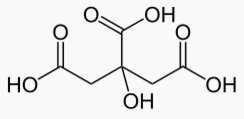
Citric C6H8O7 7.4e-4 -3.13 .185
Nitrous HNO2 5.6e-4 -3.25 .193
Formic H2CO2 1.8e-4 -3.75 .222
Acetic CH3CO2H 1.8e-5 -4.76 .281
Carbonic H2CO3 4.5e-7 -6.35 .377
Sulfide H2S 8.9e-8 -7.05 .418
Phosphoric H2PO4- 6.2e-8 -7.20 .427
Carbonic HCO3- 4.7e-11 -10.3 .611
Peroxide H2O2 2.0e-12 -11.7 .694
Phosphoric HPO4-- 4.8e-13 -12.37 .734
Water H2O 1.0e-14 -14.00 .830
All values are for 25 Celsius.
1 electron Volt = 1.602⋅10-19 Joules
Water scale = LH2O = NH2O-1/3 = 3.10⋅10-10 m size of a water molecule
Water volume = VH2O = L3H2O volume of a water molecule
Conc. relative to water = Ć = C / CH2O
Water concentration = ĆH2O = 1
Arrhenius concentration = ĆArr = 1.80⋅10-9 H+ concentration in pure water
Arrhenius scale = LArr = ĆArr-1/3 LH2O = 822 LH2O = 2.552⋅10-7 meters
Arrhenius volume = VArr = L3Arr
Arrhenius time = TArr = R-1H2O = 11.7 hours time for a water molecule to dissociate
Conc. rel to Arrhenius vol= C″ = Ć CArr
Acid const. in water units= K′ = K / CH2O molecules per water molecule
Acid const. in Arr. units = K″ = K / CH2O / CArr molecules per Arrhenius volume
Dissoc. rate in Arr. units= R″x = Rx / TArr molecules/Arrheniustime
Assoc. rate in Arr. units = R″x- = Rx- ⋅ 1000 NAvo VArr / TArr molecules/Arrheniustime
Water rate in Arr. units = R″H2O = RH2O / Tarr = 1 molecules/Arrheniustime
H+ + OH- rate in Arr. units= R″OH- = 1 molecules/Arrehniusvolume/Arrheniustime
Water const. in Arr. units= K″H2O = 1 molecules/Arrheniusvolume
moles/ molecules per molecules per
Litre water molecule Arrhenius volume
Pure water H+ concentration 10-7 1.80⋅10-9 1
Pure water H2O concentration 55.5 1 5.56⋅108
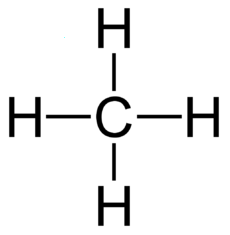
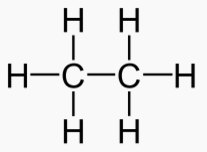

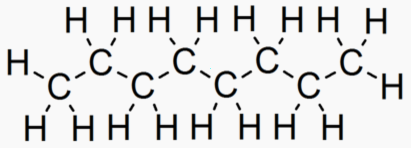
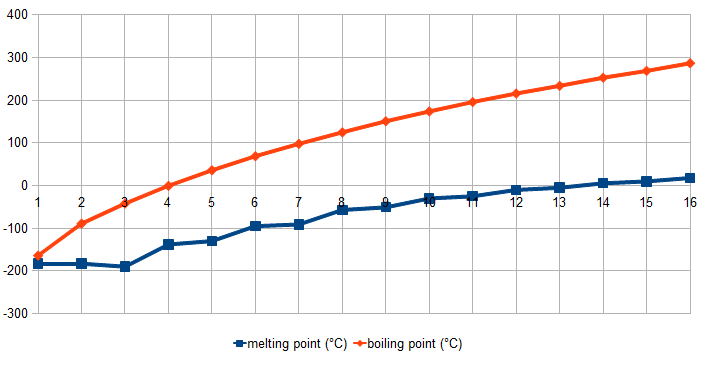
Alkane Carbons Energy of Melt Boil Solid Liquid Gas Phase at
type combustion (K) (K) density density density 300 K
(MJ/kg) (g/cm^3) (g/cm^3) (g/cm^3)
Hydrogen 0 141.8 14.0 20.3 .07 .000090 Gas
Methane 1 55.5 90.7 111.7 .423 .00070 Gas
Ethane 2 51.9 90.4 184.6 .545 .0013 Gas
Propane 3 50.4 85.5 231.1 .60 .0020 Gas
Butane 4 49.5 136 274 .60 .0025 Gas
Pentane 5 48.6 143.5 309 .63 Liquid
Hexane 6 48.2 178 342 .65 Liquid
Heptane 7 48.0 182.6 371.5 .68 Liquid
Octane 8 47.8 216.3 398.7 .70 Liquid
Dodecain 12 46 263.5 489 .75 Liquid
Hexadecane 16 46 291 560 .77 Liquid
Icosane 20 46 310 616 .79 Solid
Alkane-30 30 46 339 723 .81 Solid
Alkane-40 40 46 355 798 .82 Solid
Alkane-50 50 46 364 848 .82 Solid
Alkane-60 60 46 373 898 .83 Solid
Gasoline ~ 8 47 .76 Liquid Mostly alkanes with ~ 8 carbons
Natural gas 54 91 112 Gas Mostly methane
Coal 32 - - Solid Mostly carbon
Wood 22 - - Solid Carbon, oxygen, hydrogen
Pure carbon 1 32.8 - - Solid Pure carbon, similar to coal
Methanol 1 175.6 337.8 .79 Liquid
Ethanol 2 159 351.5 .79 Liquid
Propanol 3 147 370 Liquid
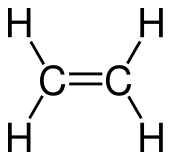
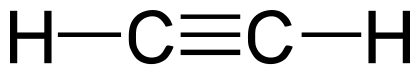
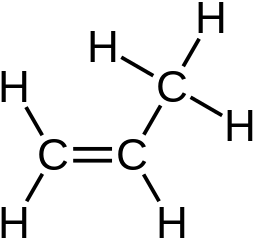
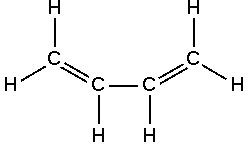
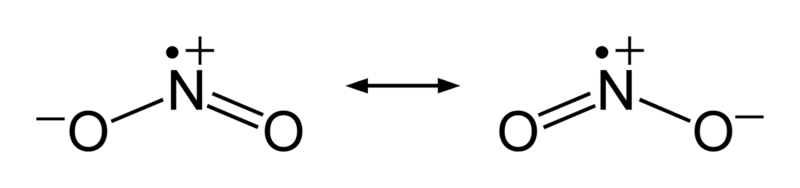
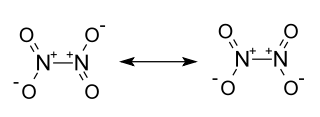
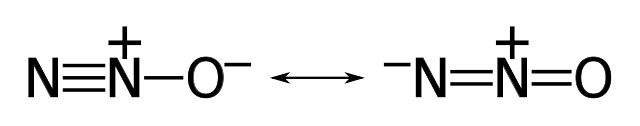
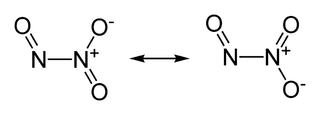
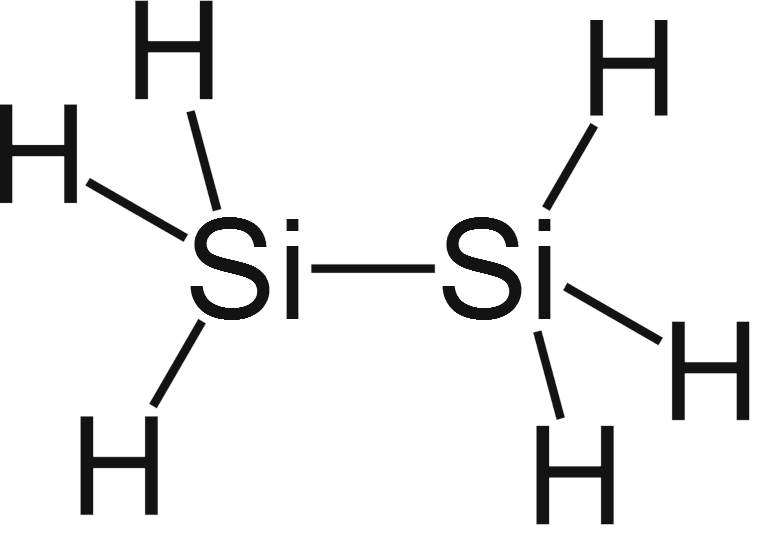
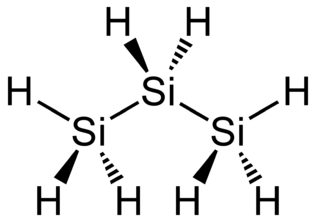
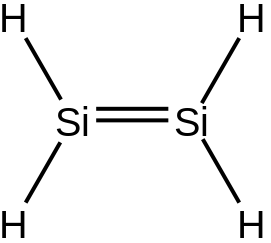
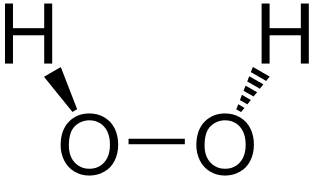
Stable Stable in an
oxygen environment
Lithium 1 1
Boron 0 0
Carbon ∞ ∞
Nitrogen 1 1
Oxygen 1 1
Aluminum 1 1
Silicon 2 0
Phosphorus 1 0
Sulfur 1 0
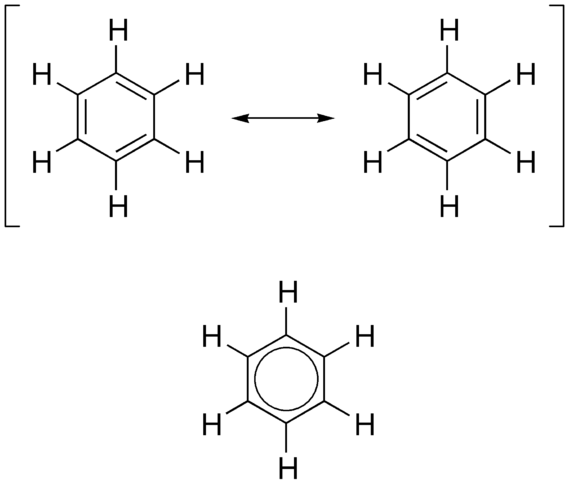
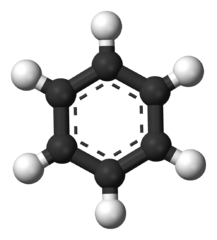
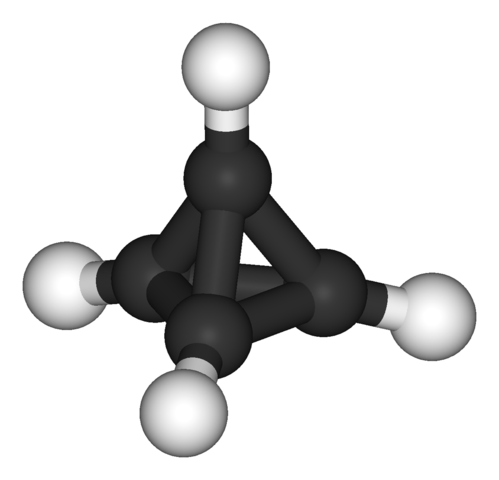
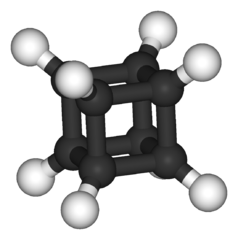

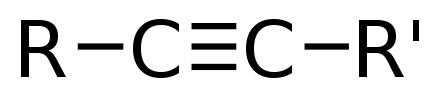
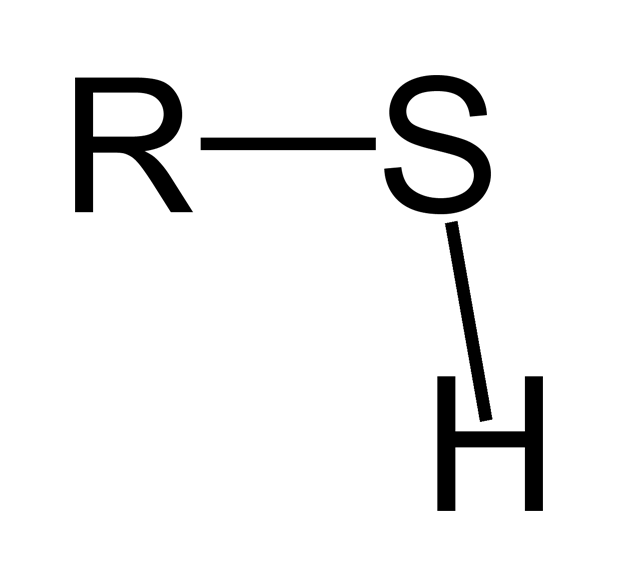
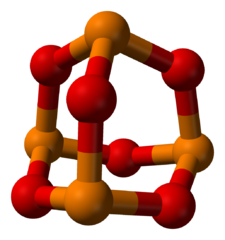
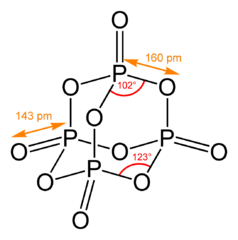
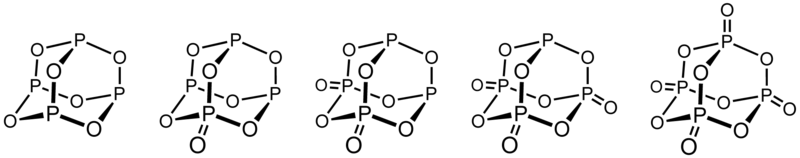
-skeletal.png)
.png)


MJoules/kg
Antimatter 90 billion
Hydrogen bomb 25000000 theoretical maximum yield
Hydrogen bomb 21700000 highest achieved yield
Uranium 20000000 as nuclear fuel
Hydrogen 143
Natural gas 53.6
Gasoline 47
Jet fuel 43
Fat 37
Coal 24
Carbohydrates & sugar 17
Protein 16.8
Wood 16
Lithium-air battery 9
TNT 4.6
Gunpowder 3
Lithium battery 1.3
Lithium-ion battery .72
Alkaline battery .59
Compressed air .5 300 atmospheres
Supercapacitor .1
Capacitor .00036
The chemical energy source with the highest energy/mass is hydrogen+oxygen, but
molecular hydrogen is difficult to harness. Hydrocarbons + oxygen is the next
best choice. Carbon offers a convenient and lightweight way to carry hydrogen
around.
MJ/kg Calories/gram
Sugar 16 5
Protein 17 5
Alcohol 25 7
Fat 38 9
Humans can metabolize a wide range of fats and sugars.
Abundance in Mass frac in
Crust (ppm) Human body
Oxygen 460000 .65
Carbon 1000 .18
Hydrogen 1500 .10
Nitrogen 20 .03
Calcium 45000 .014
Phosphorus 1100 .011
Potassium 20000 .0025
Sulfur 400 .0025
Sodium 25000 .0015
Chlorine 200 .0015
Magnesium 25000 .0005
Iron 60000 .00006
Fluorine 500 .000037
Zinc 75 .000032
Silicon 275000 .00002
Trace elements <.00001
Among the elements required for life, nitrogen is the scarcest.
The nitrogen in the first 250 km of the Earth's crust has the same mass as
the nitrogen in the atmosphere.
Used by Used by
humans bacteria
Hydrogen * *
Helium
Lithium
Beryllium
Boron * *
Carbon * *
Nitrogen * *
Oxygen * *
Fluorine *
Neon
Sodium * *
Magnesium * *
Aluminum
Silicon *
Phosphorus * *
Sulfur * *
Chlorine * *
Argon
Potassium * *
Calcium * *
Scandium
Titanium
Vanadium *
Chromium
Manganese * *
Iron * *
Cobalt * *
Nickel *
Copper * *
Zinc * *
Gallium
Germanium
Arsenic *
Selenium * *
Bromine * *
Krypton
Molybdenum *
Tellurium *
Iodine * *
Tungsten *
* Video of an amoeba
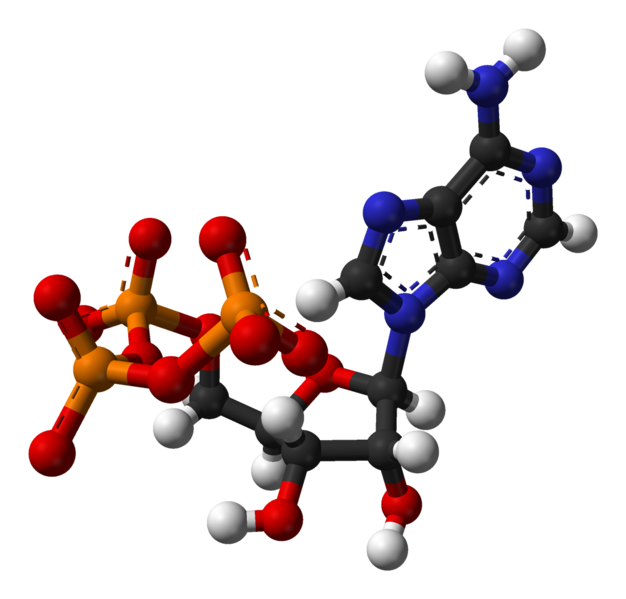
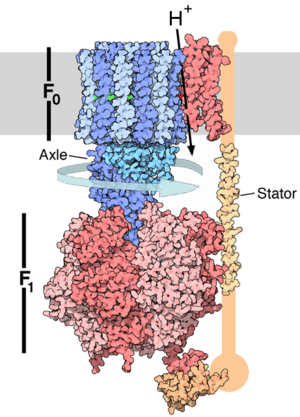
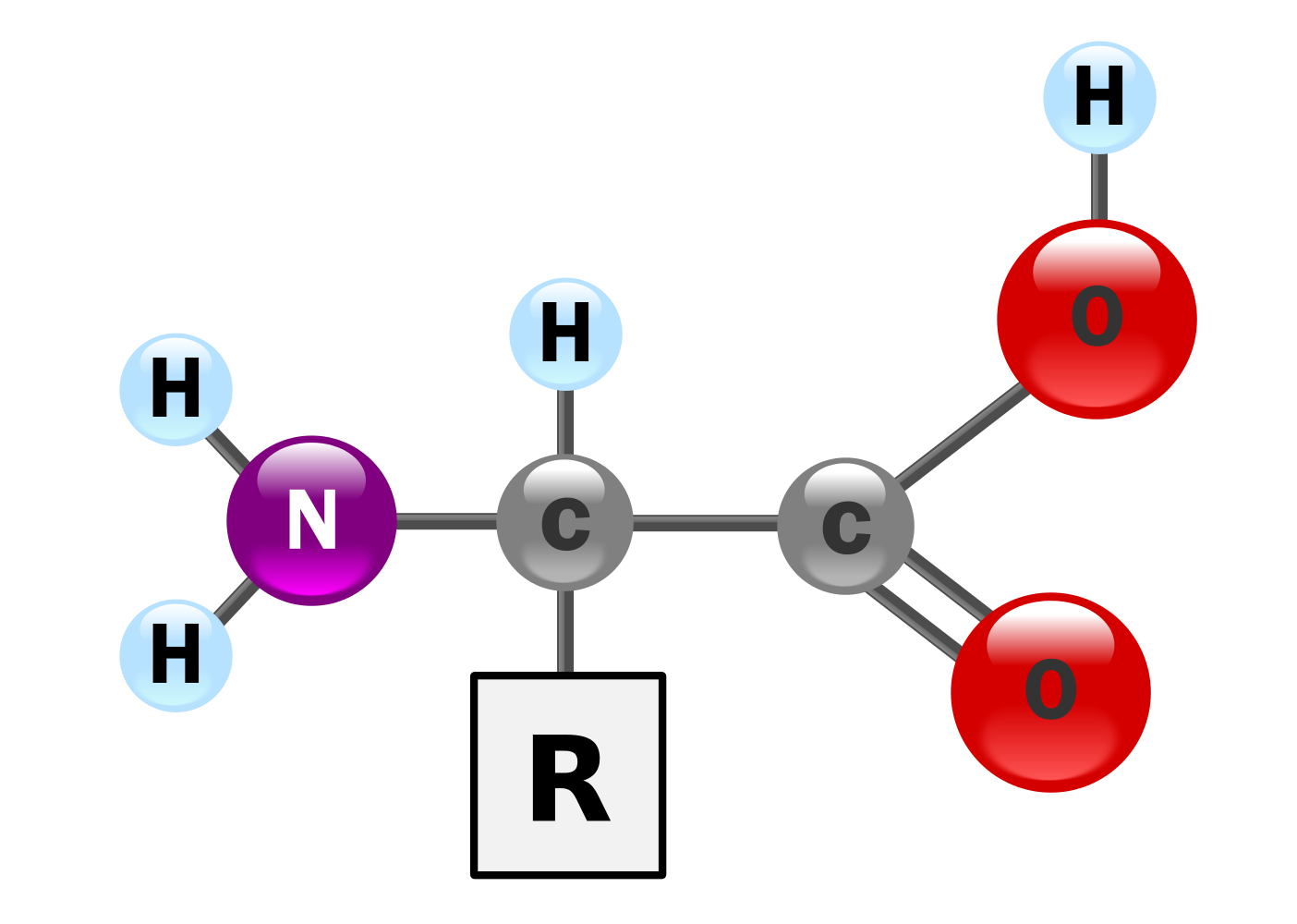
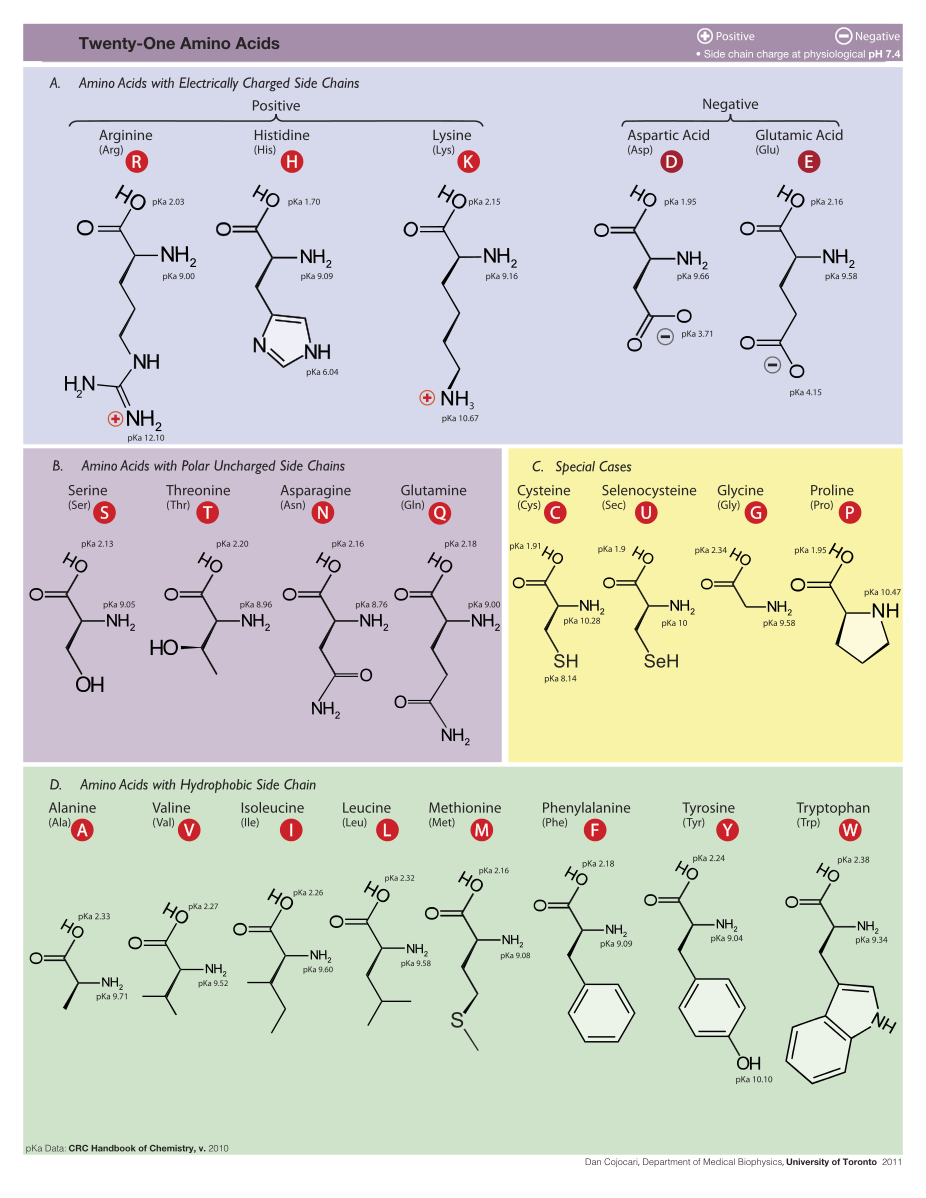
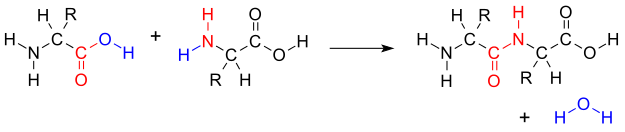
Synthesis of two amino acids. Proteins are chains of animo acids with a backbone of
the form:
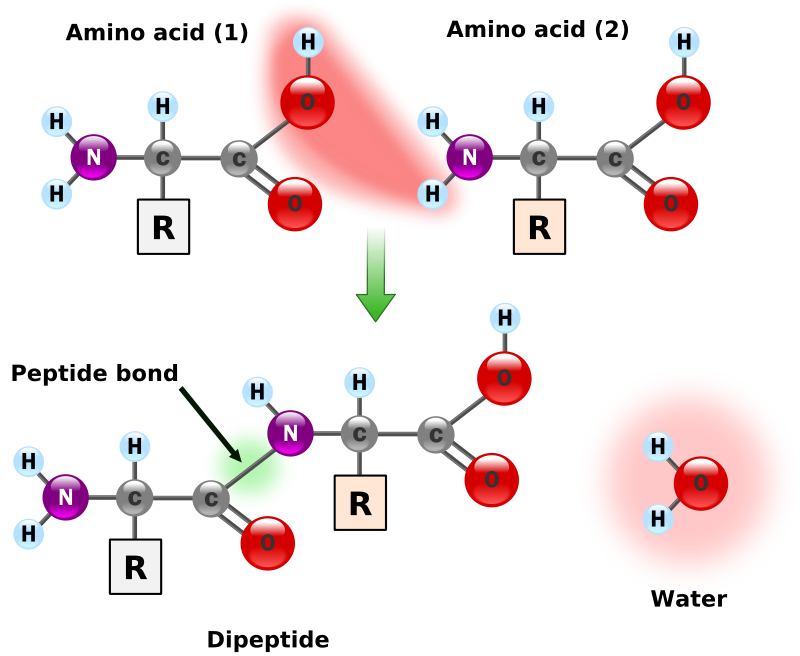
C-C-N-C-C-N-C-C-N-C-C-N-C-C-N
Number of Number of
carbons sugars
Diose 2 1
Triose 3 2
Tetrose 4 3
Pentose 5 4
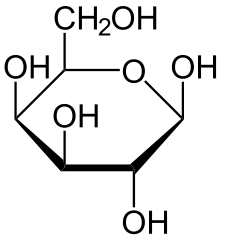
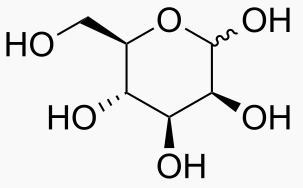
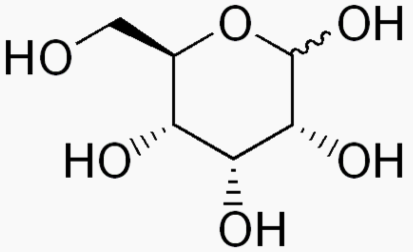
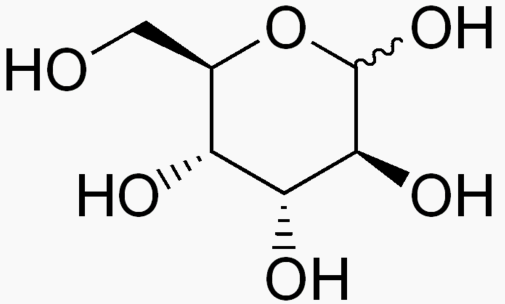
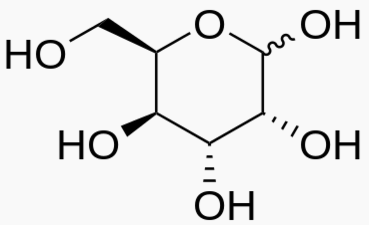
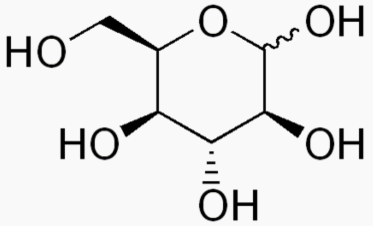
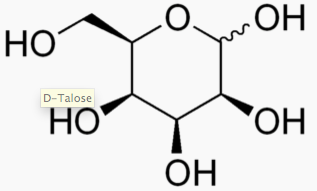
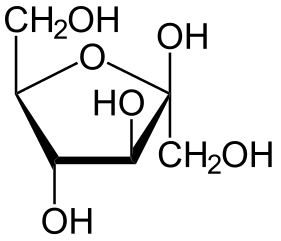
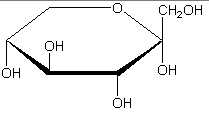
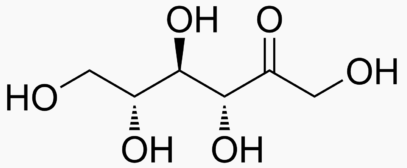
Hexose 6 12 At least 6 carbons are required to form a ring
Heptose 7 many Rarely observed in nature
Octose 8 many Unstable. Not observered in nature.
"Number of sugars" refers to the number of different types of sugar molecules for each
carbon number.
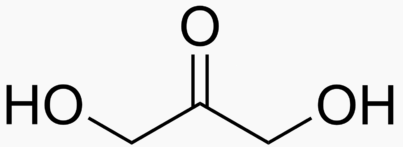
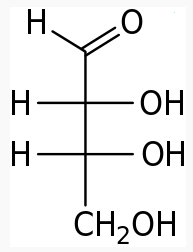
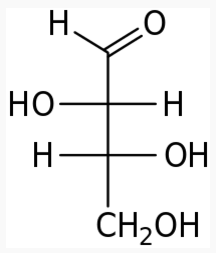
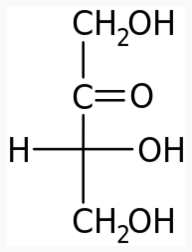
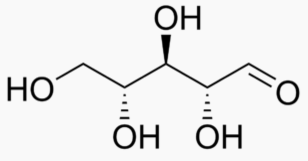
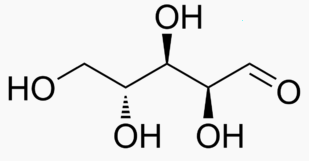
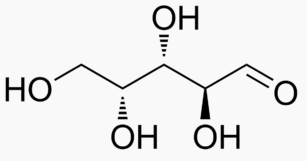
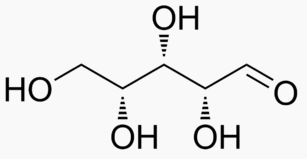

Energy Sweetness
Succrose 1.00 1.00 Benchmark
Glucose .74
Maltose .32
Galactose .32
Lactose .16
Allose
Altrose
Mannose
Fructose 1.73
Psichose .70
Tagatose .38 .92
Sorbose 1.0
Honey .97
Monosaccharde: 1 sugar molecule
Disaccharide: 2 monosaccharides
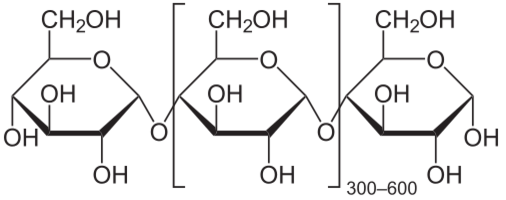
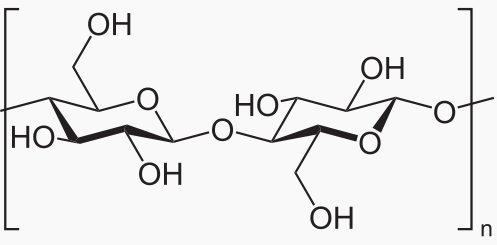
Polysaccharide: More than 2 monosaccharides, such as starch and cellulose
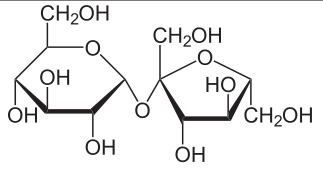
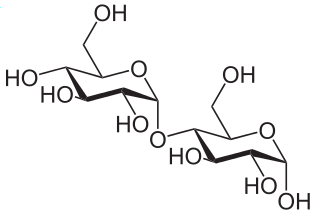
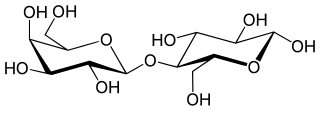
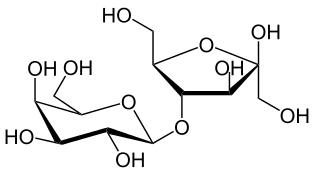
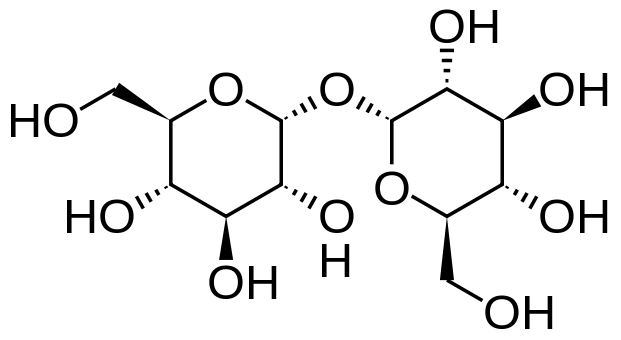
Sucrose = Glucose + Fructose
Maltose = Glucose + Glucose
Lactose = Galactose + Glucose
Lactulose = Galactoce + Fructose
Trehalose = Glucose + Glucose
Cellobiose = Glucose + Glucose
Chitobiose = Glucosamine + Glucosamine
Starch and cellulose are long chains of glucose molecules.


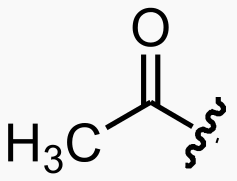
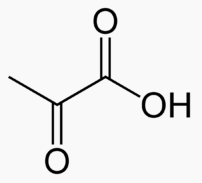
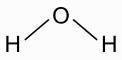
![]()
Fatty acid → Acetyl → CO2 and H2O
Sugar → Pyruvate → CO2 and H2O
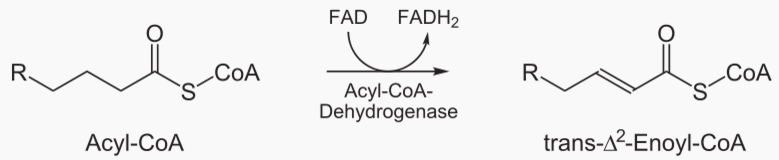
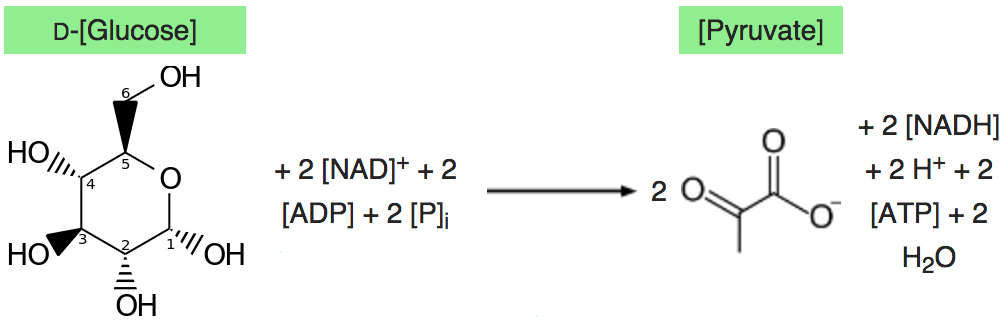
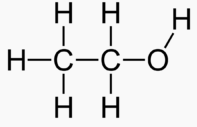
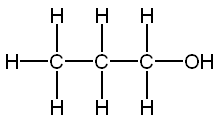
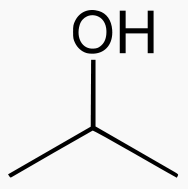
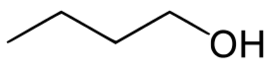
Carbons
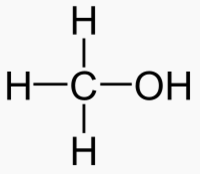
Methanol 1 Toxic
Ethanol 2 Inebriating
Propanol 3 3 times more inebriating than ethanol
Isopropanol 3 Toxic
Butanol 4 6 times more inebriating than ethanol
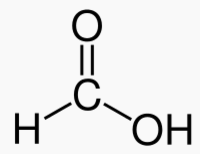
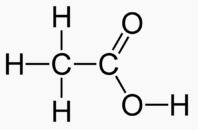

Carbons
1
2 Vinegar
3
4 Found in butter
8 Found in coconuts
10 Found in coconuts
12 Found in coconuts
16 Most common fatty acid. Found in palm oil
18 Found in chocolate
20 Found in peanut oil
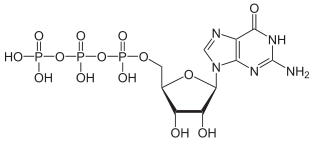
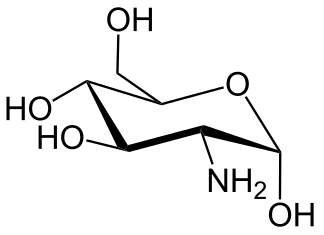


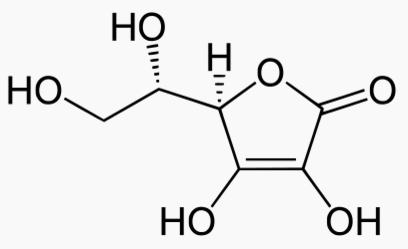
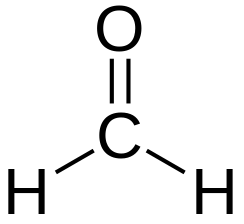
LD50
(mg/kg)
CO Carbon monoxide
HCN 6.4 Hydrogen cyanide
CH2O Methanol
CH2O Formaldehyde
H2S Hydrogen sulfide
NO2 Nitrite
Cl2 Chlorine
Fl2 Fluorine
Ethanol 7060
Salt 3000
Caffeine 192
Aspirin 200
NaNO2 180 Sodium nitrite
Cobalt 80
NaF 52
Capsaicin 47 Chili pepper
Mercury 41
Arsenic 13
Nicotine .8
Bromine
C2N2
PH3
SiCl4
Almost anything with fluorine or bromine is toxic.
C2H2 Acetylene. Inebriating
C3H6 Propene. Inebriating
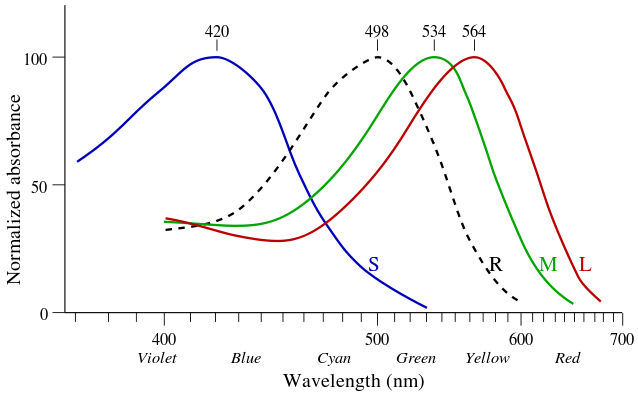
Opsin Wavelength Humans
(nm)
RH1 500 White Black/White
RH2 600 Black/White. Extinct in mammals
OPN1LW 564 Red Once possessed by mammals, then lost by most
OPN1MW 534 Green All mammals
OPN1SW 440 Blue All mammals
SWS2 480 Extinct in mammals
VA 500 Vertebrates except mammals. Vertebrae ancient opsin.
Parapinopsin UV 365 Catfish
Parapinopsin Blue 470 Catfish and lamphrey
Pareitopsin 522 Lizards
Panopsin Cyan 500 Fish vision. Found in the brains of humans
Panopsin Blue 450 Fish vision. Found in the brains of humans
Neuropsin 380 Bird vision. Found in the brains of humans
Melanopsin 480 Found in the brains of humans
Retinal G Found in the brains of humans
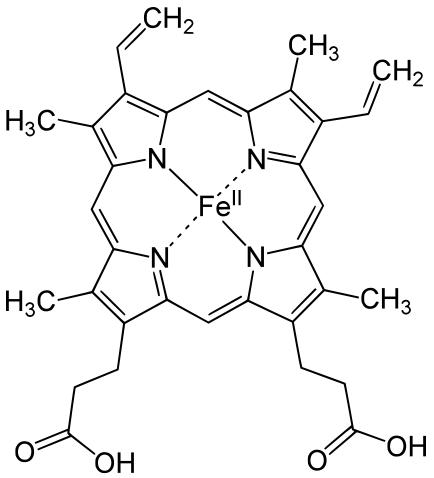
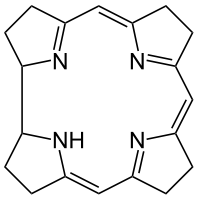
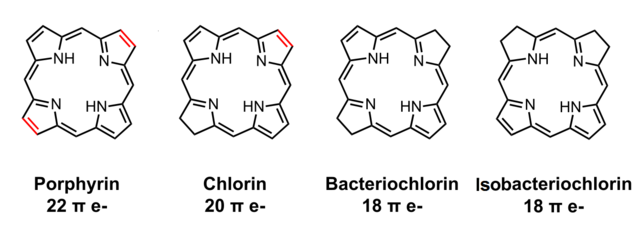
Porphin is an aromatic molecule because it is flat and because it resonates between
different electronic states.
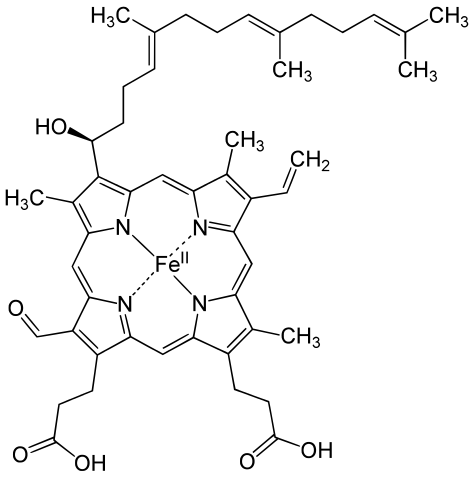

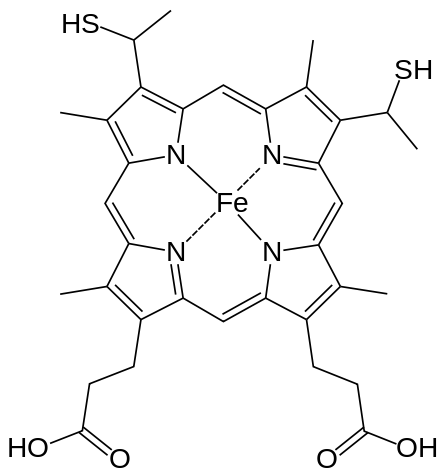
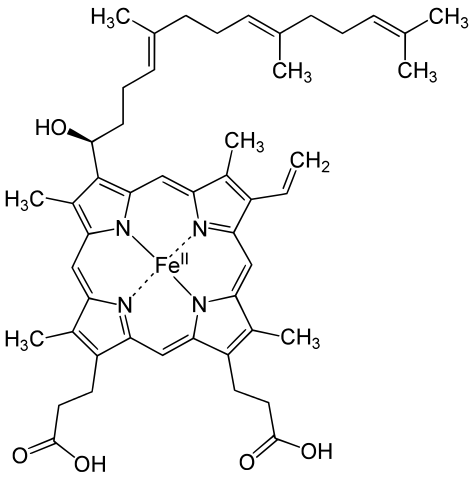
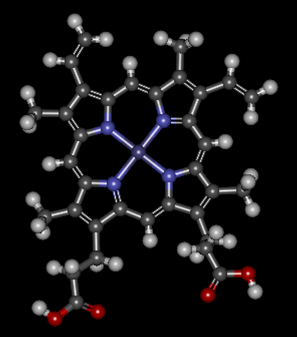
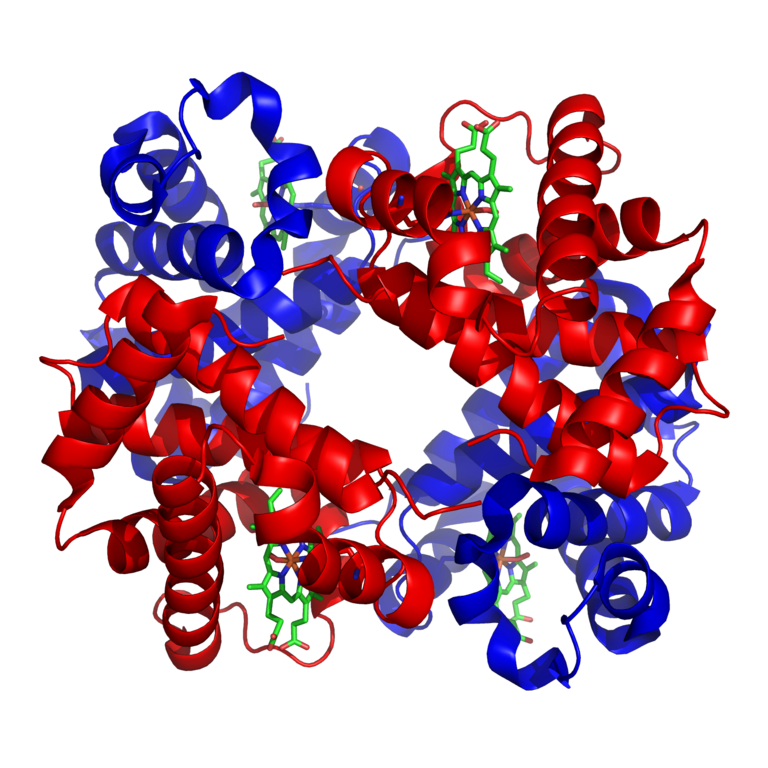
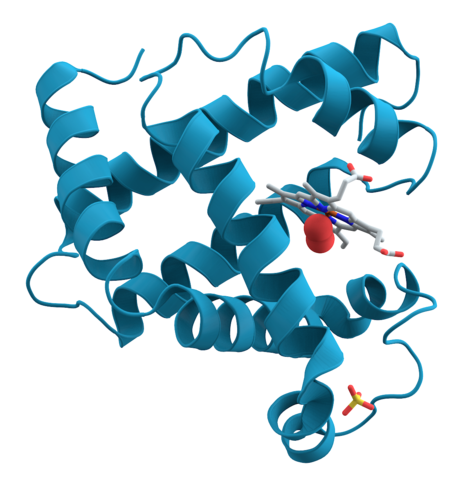
Hemoglobin is a set of 4 helix proteins that carry 4 iron ligands, and each
iron ligand carries 1 oxygen molecule.
Human hemoglobin is composed mostly of heme B.
The oxygen density of hemoglobin is 70 times the solubility of oxygen
in water.
Hemoglobin fraction of red blood cells = .96 (dry weight)
Hemoglobin fraction of red blood cells = .35 (including water)
Oxygen capacity of hemoglobin = 1.34 Liters of oxygen / kg hemoglobin
Iron ligands per hemoglobin = 4
O2 molecules per ion ligand = 1

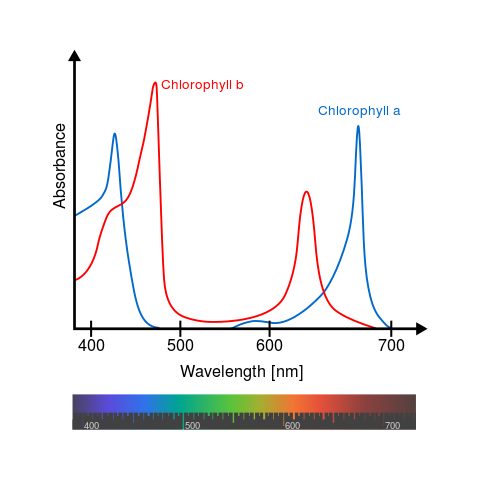
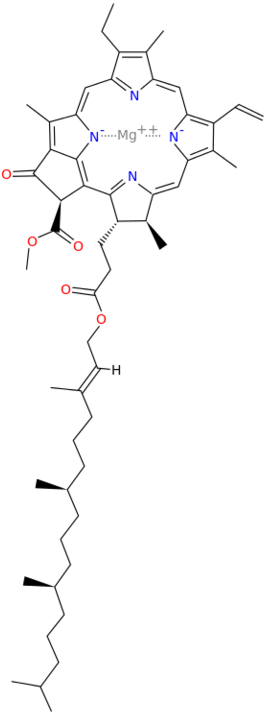
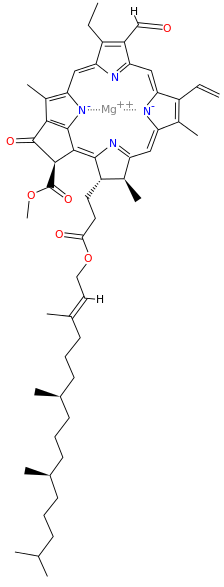
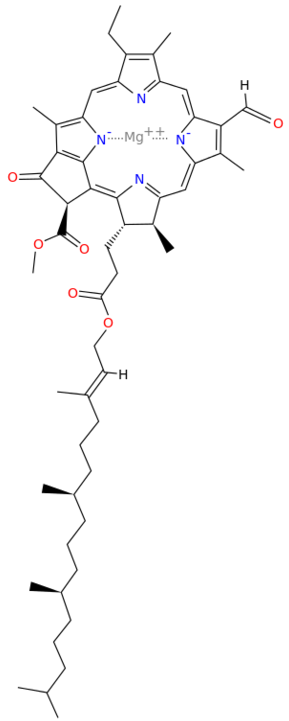
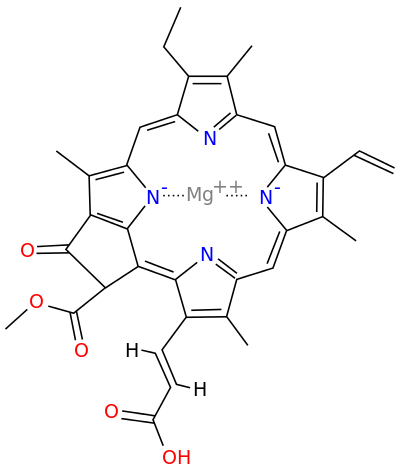
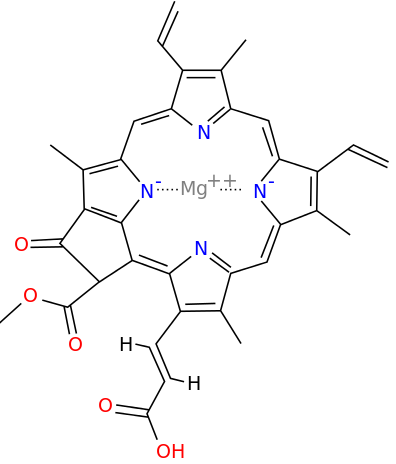
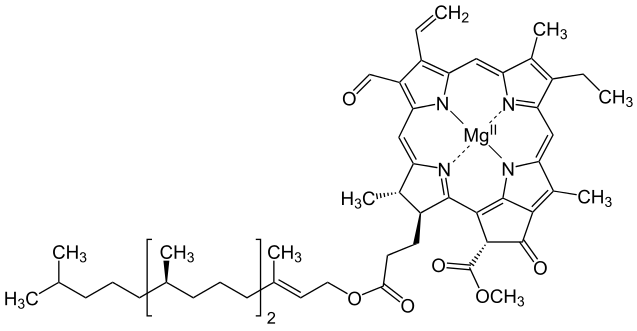
A Universal
B Plants
C1 Algae
C2 Algae
D Cyanobacteria
F Cyanobacteria
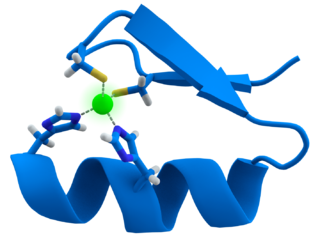
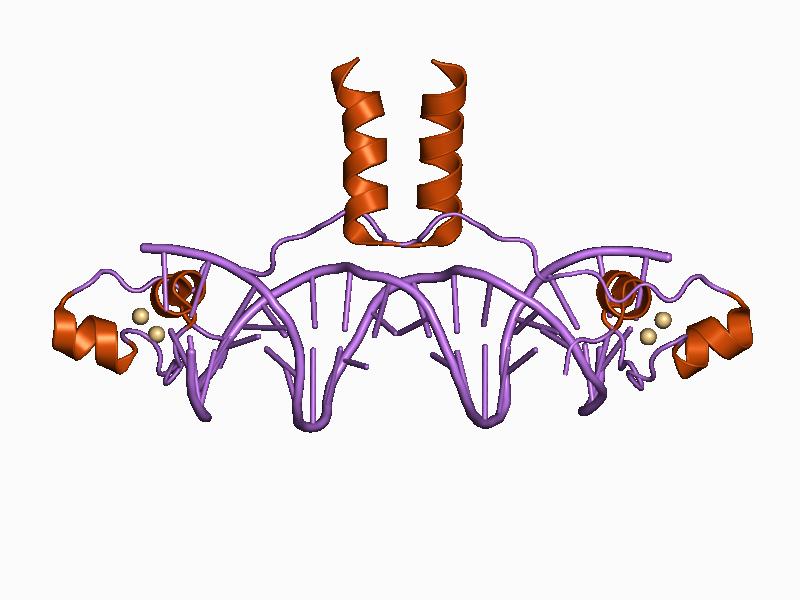
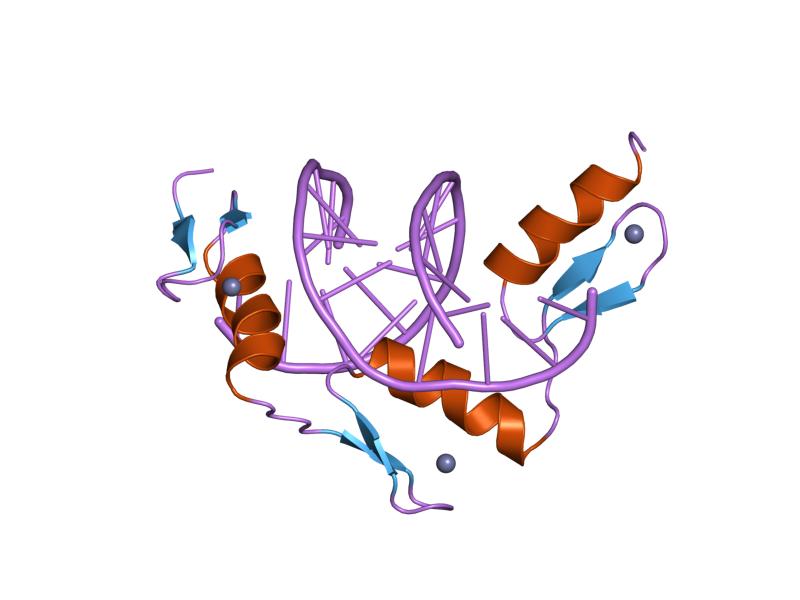
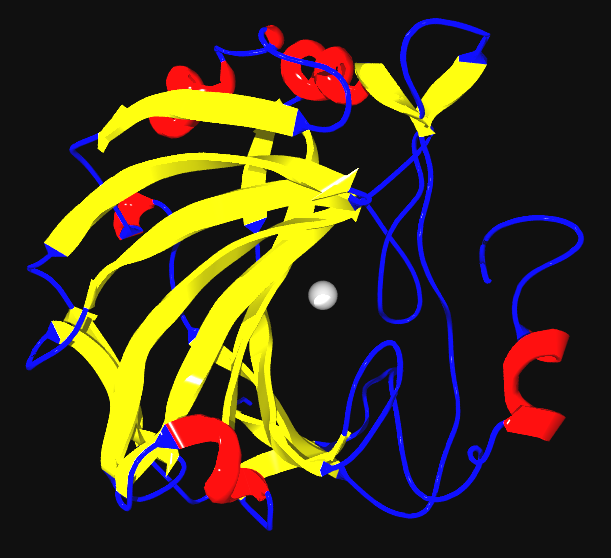
Element Humans Cofactor Function
Hydrogen *
Helium No biological role
Lithium No biological role
Beryllium Toxic becauseit displaces magnesium in proteins
Boron * Plant cell walls. Metabolism of calcium in plants & animals
Magnesium * Chlorin Chlorophyll
Scandium No biological role
Titanium No biological role
Vanadium Found only in rare bacteria.
Chromium No biological role
Manganese * Superoxide dimutase. Converts superoxide to oxygen
Iron * Porphin Hemoglobin
Cobalt * Corrin Cobalamin (Vitamin B12)
Nickel Corphin Coenzyme F430 (Creates methane. Found only in archaea)
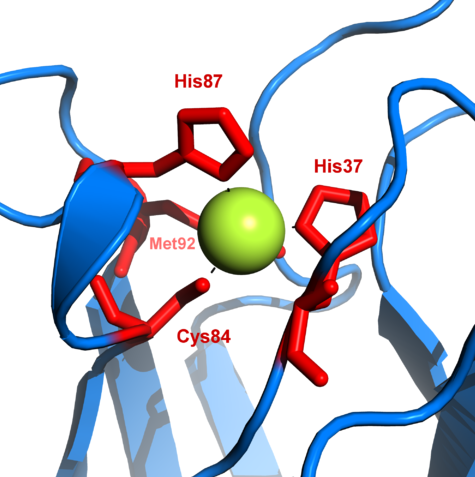
Copper * Heme Cytochrome C oxidase. Electron transport chain
Hemocyanin, an alternative to hemoglobin used by some animals
Hemoglobin carries 4 times as much oxygen as hemocyanin
Plastocyanin protein, used in photosynthesis
Sometimes used in superoxide dimutase
Zinc * Component of proteins that manipulate DNA and RNA (Zinc fingers)
Component of carbonic anhydrase, which interconverts CO2 and HCO3
Metallothionein proteins, which bind to metals such as
zinc, copper, selenium cadmium, mercury, silver, and arsenic
Molybdenum Nitrogen fixase. Convert N2 to NH3
Selenium * Component of the amino acide selenocysteine
Bromine * Limited role
Iodine * Component of thyroxine and triiodotyronine, which
regulate metabolic rate
Lead Toxic because it displaces calcium in bones




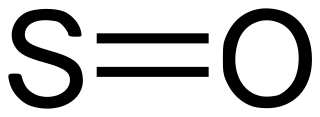
![]()













.jpg)










Alloys can be much stronger than pure metals.

Discovery Method of Source
(year) discovery
Carbon Ancient Naturally occuring
Gold Ancient Naturally occuring
Silver Ancient Naturally occuring
Sulfur Ancient Naturally occuring
Lead -6500 Smelt with carbon Galena PbS
Copper -5000 Smelt with carbon Chalcocite Cu2S
Bronze (As) -4200 Copper + Arsenic Realgar As4S4
Tin -3200 Smelt with carbon Calamine ZnCO3
Bronze (Sn) -3200 Copper + Tin
Brass -2000 Copper + Zinc Sphalerite ZnS
Mercury -2000 Heat the sulfide Cinnabar HgS
Iron -1200 Smelt with carbon Hematite Fe2O3
Arsenic 1250 Heat the sulfide Orpiment As2S3
Zinc 1300 Smelt with wool Calamine ZnCO3 (smithsonite) & Zn4Si2O7(OH)2·H2O (hemimorphite)
Antimony 1540 Smelt with iron Stibnite Sb2S3
Phosphorus 1669 Heat NaPO3 Excrement
Cobalt 1735 Smelt with carbon Cobaltite CoAsS
Platinum 1735 Naturally occuring
Nickel 1751 Smelt with carbon Nickeline NiAs
Bismuth 1753 Isolated from lead
Hydrogen 1766 Hot iron + steam Water
Oxygen 1771 Heat HgO
Nitrogen 1772 Isolated from air
Manganese 1774 Smelt with carbon Pyrolusite MnO2
Molybdenum 1781 Smelt with carbon Molybdenite MoS2
Tungsten 1783 Smelt with carbon Wolframite (Fe,Mn)WO4
Chromium 1797 Smelt with carbon Crocoite PbCrO4
Palladium 1802 Isolated from Pt
Osmium 1803 Isolated from Pt
Iridium 1803 Isolated from Pt
Rhodium 1804 Isolated from Pt
Sodium 1807 Electrolysis
Potassium 1807 Electrolysis
Magnesium 1808 Electrolysis Magnesia MgCO3
Cadmium 1817 Isolated from zinc
Lithium 1821 Electrolysis of LiO2 Petalite LiAlSi4O10
Zirconium 1824 Smelt with potassium Zircon ZrSiO4
Aluminum 1827 Smelt with potassium
Silicon 1823 Smelt with potassium
Beryllium 1828 Smelt with potassium Beryl Be3Al2Si6O18
Thorium 1929 Smelt with potassium Gadolinite (Ce,La,Nd,Y)2FeBe2Si2O10
Vanadium 1831 Smelt VCl2 with H2 Vanadinite Pb5(VO4)3Cl
Uranium 1841 Smelt with potassium Uranite UO2
Ruthenium 1844 Isolated from Pt
Tantalum 1864 Smelt with hydrogen Tantalite [(Fe,Mn)Ta2O6]
Niobium 1864 Smelt with hydrogen Tantalite [(Fe,Mn)Ta2O6]
Fluorine 1886 Electrolysis
Helium 1895 From uranium ore
Titanium 1910 Smelt with sodium Ilmenite FeTiO3
Hafnium 1924 Isolated from zirconium
Rhenium 1928 Isolated from Pt
Scandium 1937 Electrolysis Gadolinite FeTiO3
-384 -322 Aristotle. Wrote "Meteorology"
-370 -285 Theophrastus. Wrote "De Mineralibus"
77 Pliny the Elder publishes "Natural History"
973 1050 Al Biruni. Published "Gems"
1546 Georgius Agricola publishes "On the Nature of Rocks"
1556 Georgius Agricola publishes "On Metals"
1609 de Boodt publishes a catalog of minerals
1669 Brand: Discovery of phosphorus
1714 John Woodward publishes "Naturalis historia telluris illustrata & aucta", a mineral catalog
1735 Brandt: Discovery of cobalt
1777 Lavoisier: Discovery of sulfur
1778 Lavoisier: Discovery of oxygen and prediction of silicon
1783 Lavoisier: Discovery of hydrogen
1784 T. Olof Bergman publishes "Manuel du mineralogiste, ou sciagraphie du regne mineral",
and founds analytical chemistry
1778 Lavoisier: Discovery of oxygen
1801 Rene Just Huay publishes "Traite de Mineralogie", founding crystallography
1811 Avogadro publishes "Avogadro's law"
1860 The Karlsruhe Congress publishes a table of atomic weights
1869 Mendeleev publishes the periodic table
Shear modulus = S = 82 GJoules/meter3
Density = D = 7900 kg/meter3
Sword worthiness = Q = S/D = 10.4 MJoules/kg


-600 Wootz steel developed in India and is renowned as the finest steel in the world.
1700 The technique for making Wootz steel is lost.
1790 Wootz steel begins to be studied by the British Royal Society.
1838 Anosov replicates Wootz steel.
Wootz steel is a mix of two phases: martensite (crystalline iron with .5% carbon),
and cementite (iron carbide, Fe, 6.7% carbon).




Yield strength = Y (Pascals)
Density = D (kg/meter3)
Quality = Q = Y/D (Joules/kg)
The strongest allyos are:
Yield strength Density Quality
(GPa) (g/cm3) (MJoule/kg)
Magnesium + Lithium .14 1.43 98
Magnesium + Y2O3 .31 1.76 177
Aluminum + Beryllium .41 2.27 181
Amorphous LiMgAlScTi 1.97 2.67 738
Diamond 1.6 3.5 457
Titanium + AlVCrMo 1.20 4.6 261
Amorphous AlCrFeCoNiTi 2.26 6.5 377
Steel + Cobalt, Nickel 2.07 8.6 241
Amorphous VNbMoTaW 1.22 12.3 99
Molybdenum+ Tungsten, Hafnium 1.8 14.3 126
The strongest pure metals are weaker than the strongest alloys.
Yield strength Density Quality
(GPa) (g/cm3) (MJoule/kg)
Magnesium .10 1.74 57
Beryllium .34 1.85 184
Aluminum .02 2.70 7
Titanium .22 4.51 49
Chromium .14 7.15 20
Iron .10 7.87 13
Cobalt .48 8.90 54
Molybdenum .25 10.28 24
Tungsten .95 19.25 49
Beryllium + Li → Doesn't exist. The atoms don't mix
Beryllium + Al → Improves strength
Magnesium + Li → Weaker and lighter than pure Mg. Lightest existing alloy
Magnesium + Be → Only tiny amounts of beryllium can be added to magnesium
Magnesium + Carbon tubes → Improves strength, with an optimal tube fraction of 1%
Aluminum + Li,Mg,Be,Sc → Stronger and lighter than aluminum
Titanium + Li,Mg,Sc → Stronger and lighter than titanium
Steel + Cr,Mo → Stronger and more uncorrodable than steel. "Chromoly"
Copper + Be → Stronger than beryllium and is unsparkable
Melting point (Celsius)
Tungsten 3422
Rhenium 3186
Osmium 3033
Tantalum 3017
Molybdenum 2623
Niobium 2477
Iridium 2446
Ruthenium 2334
Hafnium 2233
Technetium 2157
Rhodium 1964
Vanadium 1910
Chromium 1907
20 600 800 900 1000 1100 1200 1400 1600 1800 1900 Celsius
VNbMoTaW 1.22 .84 .82 .75 .66 .48 .4
AlMohNbTahTiZr 2.0 1.87 1.60 1.2 .74 .7 .25
Nickel superalloy 1.05 1.20 .90 .60 .38 .15
Tungsten .95 .42 .39 .34 .31 .28 .25 .10 .08 .04
Below 1100 Celsius AlMohNbTahTiZr has the best strength-to-mass ratio and above
this VNbMoTaW has the best ratio. Both alloys supersede nickel superalloy
and both outperform tungsten, the metal with the highest melting point.
Data:
Entropy, nickel superalloy
Yield strength (GPa)
Copper .27
Brass .41 30% zinc
Bronze .30 5% tin
Phosphor bronze .69 10% tin, .25% phosphorus
Copper + beryllium 1.2 2% beryllium, .3% cobalt
Copper + nickel + zinc .48 18% nickel, 17% zinc
Copper + nickel .40 10% nickel, 1.25% iron, .4% manganese
Copper + aluminum .17 8% aluminum



Atom Form of Atomization energy
raw element kJoules/mole
H H2 218
He He 0 Noble elements are already atomized
Be Metal 159
Li Metal 324
B Boron solid 563
C Graphite 717
N N2 473
O O2 249
F F2 79
Ne Ne 0
Na Metal 107
Mg Metal 146
Al Metal 326
Si Crystal 456
P Solid 315
S Solid 279
Cl Cl2 122
Fe Metal 415
Pressure = P
Volume = V
Entropy = S
Temperature = T
Internal energy = E
Enthalpy = H = E + P V
Gibbs energy = G = E + P V - T S
Helmholtz energy = A = E - T S
The Gibbs energy is the energy required to assemble a molecule from raw
elements at standard temperatue (298.2 Kelvin) and pressure (1 bar). The Gibbs energy
of raw elements is defined as zero.
Energy = E Joules
Electron volt = e = 1.602e-19 Joules = 96.47 kJoules/mole
Avogadro number = A = 6.0221e23 particles/mole
Atomic mass unit = m = 1.6605e-27 kg
Boltzmann constant = k = 1.3806e-23 Joules/Kelvin
H2O → H2 + ½ O2 - 286 kJ/mole
→ 2 H + O - 286 - 2*218 - 249 kJ/mole
→ 2 H + O - 971 kJ/mole
Gibbs Enthalpy Entropy Atomize
kJ/mole kJ/mole kJ/mole/K kJ/mole
H2 0 0 .131 -436
C graphite 0 0 .00574 -717
N2 0 0 .1915 -946
O2 0 0 .2050 -498
H2O -237.24 -285.83 .06995 -716 -286 - 2*218 - 249
CO2 -394.4 -393.5 .214 -1609 -394 - 717 - 498
CH4 -74.87 .1862 -1664 -75 - 717 - 872
C2H6 -83.7
C3H8 -104.6
C4H10 -125.5
C5H12 -146.9
C6H14 -167.4
C7H16 -187.9
C8H18 -208.4
C12H26 -352.1 .4907
C16H34 -456.3 .5862
Gunpowder has oxygen in the mixture in the form of
KNO3 which makes it burn faster.
eV kJoules/mole
H - H
HO - H 493.4
O - H 424.4
CH3 - H 4.52 435
CH2 - H 444
CH - H 444
C - H 339
C2H5 - H 423
C2H - H 556
C6H5 - H 473
CH3 - CH3 3.64 351
CH2 = CH2 622
CH ≡ CH 837
C2H3 - H 464
CH2CHCH2 - H 372
Gibbs Enthalpy Entropy
kJoule/mole kJoule/mole kJoule/mole/K
H2 0 0 .131
C graphite 0 0 .00574
C diamond 2.90 1.90 .00238
N2 0 0 .1915
O2 0 0 .2050
Ne 0 0 .1464
Na 0 0 .0512
Mg 0 0 .0327
Al 0 0 .0283
Al 0 0 .0283
Si 0 0 .0188
P4 0 0 .1644
S 0 0 .0318
Cl2 0 0 .223
K 0 0 .0642
Ca 0 0 .0414
Ar 0 0 .155
Cr 0 0 .0238
Mn 0 0 .0327
Fe 0 0 .0273
Ni 0 0 .0299
Cu 0 0 .0332
Zn 0 0 .0416
Ag 0 0 .0426
Sn 0 0 .0516
Hg 0 0 .0760
Pb 0 0 .0648
H2O -237.24 -285.83 .06995
CH4 -74.87 .1862 Combustion = -890.7
C2H6 -83.7 Combustion = -1561
C3H8 -104.6
C4H10 -125.5
C5H12 -146.9
C6H14 -167.4
C7H16 -187.9
C8H18 -208.4
C12H26 -352.1 .4907 Combustion =-7901.7
C16H34 -456.3 .5862 Combustion =-10699
N2O 82.05 .2200
H2O2 -187.80
N2O4 9.16 .3043
N2H4 50.63 .1215
NH4NO3
NH4ClO4
HNO3 -207 .146
Li2O -561.9 -20.01 .03789
C4H8N8O8 HMX explosive
BeO -579.1
B2O3 -1184
CO2 -394.4 -393.5 .214
CO -137.2 -110.5 .198
NO 90.2 .211
NO2 33.2 .240
Na2O -377
MgO -596.3 -601.6 .0269
Al2O3 -1582.3 -1675.7 .0509
SiO2 -856.6 -910.9 .0418 Quartz
P2O5
SO2 -296.8 .2481
SO3 -395.7 .2567
K2O -322.2
CaO -533.0 -634.9 .0398
TiO2 -852.7
Ti2O3 -1448
VO -404.2
V2O3 -1139.3
V2O4 -1318.4
Cr2O3 -1053.1 -1139.7 .0812
MnO2 -465.2
MnO -362.9 -385.2 .0597
MnO2 -520.0 .0530
Fe2O3 -741.0 -824.2 .0874
Fe3O4 -1014 -1118.4 .0146
CoO -214.2
Co3O4 -795.0
NiO -211.7 -239.7 .0380
CuO -129.7 -157.3 .0426
Cu2O -146.0 -168.6 .0931
ZnO -318.2 -350.5 .0436
MoO2 -533.0
MoO3 -668.0
Ag2O -11.2
PdO
SnO2 -577.6 .0523
CdO -228.4
WO2 -533.9
WO3 -764.1
HgO -58.5 -90.8 .0703
PbO -219.0 .0665
PbO2 -219.0 -277.4 .0686
UO2
CuS -53.1 .0665
Cu2S -79.5 .1209
ZnS2 -206.0 .0577
CaCO3 -1128 -1207 .090
H+ (aq) 0 0 0
OH- (aq) -157.2 -230.0 -.0108
H2O (l) -237.2 -285.8 .0699
H2O (g) -228.6 -241.8 .1888
Cu+ 71.7 .0406
Cu+2 64.8 -.0996
Ca+2 -543.0 -.0531
Ag+ (aq) 105.8 .0727
Al+3 (aq) -538.4 -.3217
CO3-2 -675.2 -.0569
NH3 (g) -16.4 -46.1
NaCl (s) -384.1 -411.2 .0721
Gibbs Enthalpy Entropy
kJoule/mole kJoule/mole kJoule/mole/K
Target Molecule Bonds Bond 1st 2nd 3rd 4th
atom energy bond bond bond bond
(eV) (eV) (eV) (eV) (eV)
H H2 1 4.52 4.52
He - 0 - -
Li LiH 1 2.56 2.56
Be BeH2 2 ? 2.35 ?
B BH3 3 ? 3.43 ? ?
C CH4 4 4.31 3.52 4.61 4.61 4.52
N NH3 3 3.90 3.26 3.91 4.52
O H2O 2 4.76 4.41 5.12
F HF 1 5.90 4.90
Ne - 0 - -
Na NaH 1 2.09 2.09
Mg MgH2 2 ? 2.04 ?
Al AlH3 3 ? 2.96 ? ?
Si SiH4 4 ? 3.10 ? ? 4.08
P PH3 3 ? 3.56 ? ?
S H2S 2 3.76 3.57 3.95
Cl HCl 1 4.48 4.48
Ar - 0 - -
K KH i 1.90 1.90
Ca CaH2 i ? 1.74 ?
Ga ? ? ? ?
Ge ? 3.36 ? ? ?
As ? 2.82 ? ?
Se SeH2 2 ? 3.17 ?
Br HBr 1 3.80 3.80
Kr - 0 - -
In ? ? ? ?
Sn ? 2.77 ? ? ?
Sb ? ? ? ?
Te TeH2 2 ? 2.78 ?
I HI 1 3.10 3.10
Xe - 0 - -
Single Double Triple Quadruple
B B 3.04
B C 3.69
B O 5.56
C C 3.65 6.45 8.68 6.32
C N 3.19 6.38 9.19
C O 3.73 7.7 11.11
C Si 3.30
C P 2.74
C S 2.82 5.94
N N 1.76 4.33 9.79
N O 2.08 6.29
N Si 3.70
N P
N S
O O 1.50 5.15
O Si 4.69
O P 3.47 5.64
O S 5.41
Si Si 2.30
Si S 3.04
Si P
P P 2.08
P S 3.47
S S 2.34 4.41
H H 4.52
H C 4.25
H N 4.05
H O 3.79
H F 5.89
H Si 3.30
H P 3.34
H S 3.76
H Cl 4.48
Enthalpy
kJ/mole
H2O -285.83
Li2O -20.01
BeO -609.4
CO2 -393.5
MgO -601.6
Al2O3 -1675.7
Fe2O3 -824.2
Enthalpy Entropy Boil Density
kJ/mole kJ/mole Kelvin
O2 0 .2050
O3 142.67 .2389 161
H2O2 -187.80
NO 90.2 .211
NO2 33.2 .240
HNO3 -207 .146 356 1.51
N2O3 91.20 .3146 277 1.45
N2O4 9.16
N2O5 -43.1 .1782 320 1.64 solid
N2O 82.05 .2200
SO2 -296.8 .2481 265
SO3 -395.7 .2567
N2H4 50.63 .1215
NH4NO3 -365.6 483 1.72
NH4ClO4 -295.77 .1842
C4H8N8O8 296.16 HMX explosive
NH3OHNO3



Cu2O + C → 2 Cu + CO
At low temperature copper stays in the form of Cu2O and at high
temperature it gives the oxygen to carbon and becomes pure copper.
3 Fe2O3 + C → 2 Fe3O4 + CO
Fe3O4 + C → 3 FeO + CO
FeO + C → Fe + CO
Oxidation state = Number of electrons each iron atom gives to oxygen
Oxidation state
CuO 2
Cu2O 1
Cu 0
Fe2O3 3
Fe3O4 8/3
FeO 2
Fe 0
Smelt Method Year Abundance
(C) (ppm)
Gold <0 * Ancient .0031
Silver <0 * Ancient .08
Platinum <0 * 1735 .0037
Mercury <0 heat -2000 .067
Palladium <0 chem 1802 .0063
Copper 80 C -5000 68
Sulfur 200 * Ancient 420
Lead 350 C -6500 10
Nickel 500 C 1751 90
Cadmium 500 C 1817 .15
Cobalt 525 ? 1735 30
Tin 725 C -3200 2.2
Iron 750 C -1000 63000
Phosphorus 750 heat 1669 10000
Tungsten 850 C 1783 1100
Potassium 850 e- 1807 15000
Zinc 975 C 1746 79
Sodium 1000 e- 1807 23000
Chromium 1250 C 1797 140
Niobium 1300 H 1864 17
Manganese 1450 C 1774 1120
Vanadium 1550 ? 1831 190
Silicon 1575 K 1823 270000
Titanium 1650 Na 1910 66000
Magnesium 1875 e- 1808 29000
Lithium 1900 e- 1821 17
Aluminum 2000 K 1827 82000
Uranium 2000 K 1841 1.8
Beryllium 2350 K 1828 1.9
Smelt: Temperature required to smelt with carbon
Method: Method used to purify the metal when it was first discovered
*: The element occurs in its pure form naturally
C: Smelt with carbon
K: Smelt with potassium
Na: Smelt with sodium
H: Smelt with hydrogen
e-: Electrolysis
heat: Heat causes the oxide to decompose into pure metal. No carbon required.
chem: Chemical separation
Discovery: Year the element was first obtained in pure form
Abundance: Abundance in the Earth's crust in parts per million
Elements with a low carbon smelting temperature were discovered in ancient
times unless the element was rare. Cobalt was discovered in 1735, the first new
metal since antiquity, and this inspired scientists to smelt every known
mineral in the hope that it would yield a new metal. By 1800 all the rare
elements that were carbon smeltable were discovered.

Fe2O3 + 2 Al → 2 Fe + Al2O3
Oxidation state Oxidation state
at left at right
2 M2O ↔ 4 M + O2 1 0
4 MO ↔ 2 M2O + O2 2 1
2 M3O4 ↔ 6 MO + O2 8/3 2
6 M2O3 ↔ 4 M3O4 + O2 3 8/3
2 M2O3 ↔ 4 MO + O2 3 2
2 MO ↔ 2 M + O2 2 0
2/3 M2O3 ↔ 4/3 M + O2 3 0
1 MO2 ↔ 1 M + O2 4 0
2 MO2 ↔ 2 MO + O2 4 2
.jpg)













2(OH)6.jpg)













_.jpg)







.jpg)






.jpg)






_3.jpg)












-178918.jpg)















































































































Hydrogen White
Carbon Black
Nitrogen Blue
Oxygen Red
Sulfur Yellow
Scoville scale (relative capsaicin content)
Ghost pepper 1000000
Trinidad 1000000 Trinidad moruga scorpion
Naga Morich 1000000
Habanero 250000
Cayenne pepper 40000
Malagueta pepper 40000
Tabasco 40000
Jalapeno 5000
Guajillo pepper 5000
Cubanelle 500
Banana pepper 500
Bell pepper 50
Pimento 50
Molecule Relative hotness
Rresiniferatoxin 16000
Tinyatoxin 5300
Capsaicin 16 Chili pepper
Nonivamide 9.2 Chili pepper
Shogaol .16 Ginger
Piperine .1 Black pepper
Gingerol .06 Ginger
Capsiate .016 Chili pepper
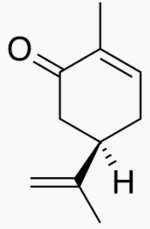

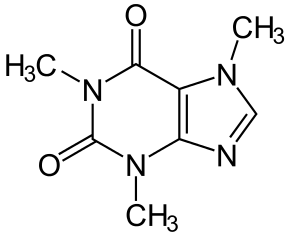
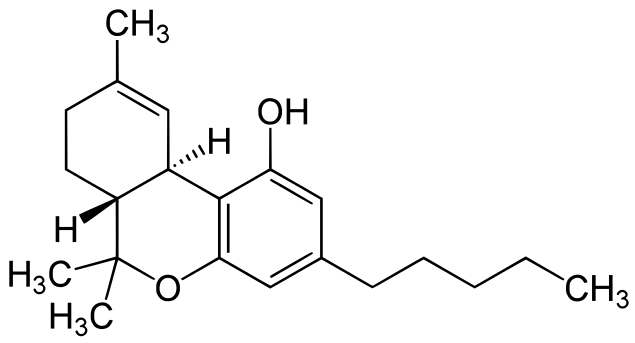
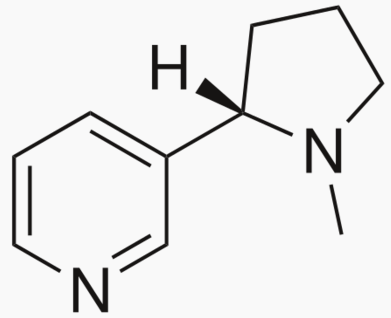
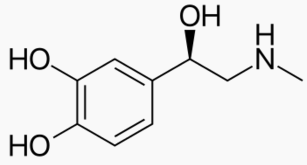
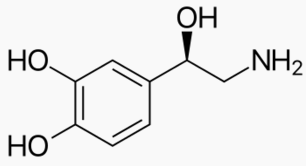
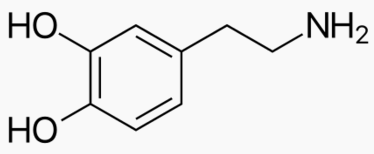
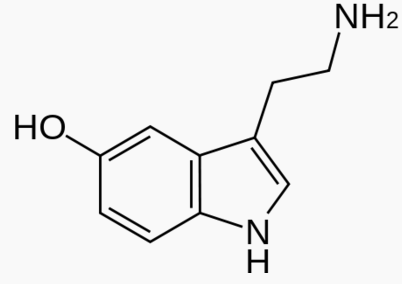
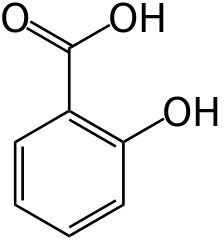
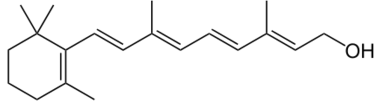


Strength Half life Dose
hours mg
Carfentanil 30000 7.7 .0003
Ohmefentanil 6300
Dihydroetorphine 4000 .03
Etorphine 2000 .006
Sufentanil 750 4.4 .015
Ocfentanil 180 .06
Fentanyl 75 .04 .1
Oxymorphone 7 8 10
Hydromorphone 5 2.5 1.5 Dilaudid
Heroine 4.5 <.6 2.2 Diamorphine
Methadone 3.5 30 35
Oxycodone 1.5 4 6.7
Morphine 1 2.5 10
Hydrocodone 1 5 10 Vicodin
Codeine .1 2.8 180
Naproxen .0072 18 1380
Ibuprofen .0045 2 2220
Aspirin .0028 6 3600
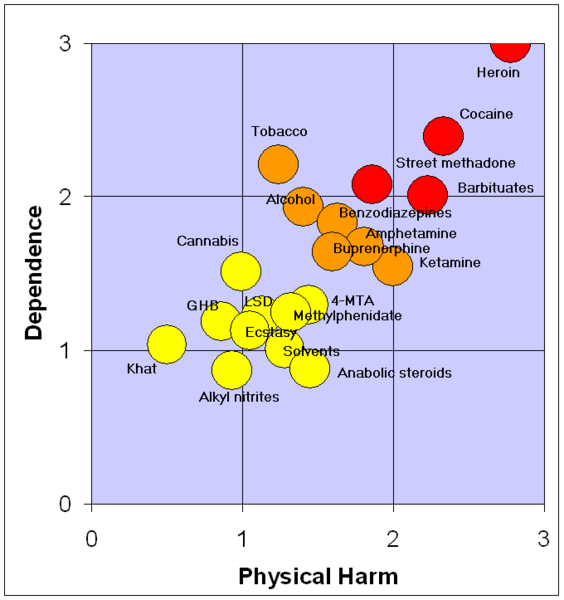
% First isolated
Morphine 10 1817 Used to produce heroine
Codeine 2 1832
Thebaine 8 Used to produce hydrocodone and hydromorphone
Papaverine 14 1848 Not psychoactive
Noscapine 5 1820 Not psychoactive
Other alkaloiods .1
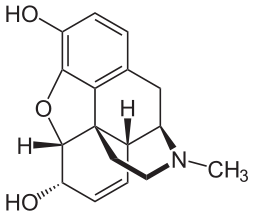
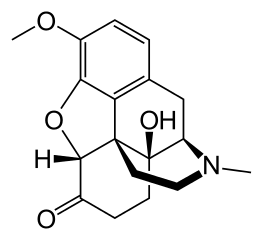
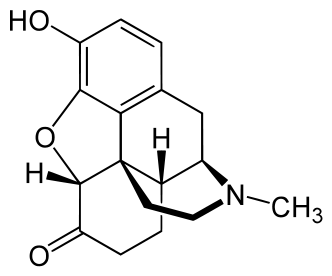
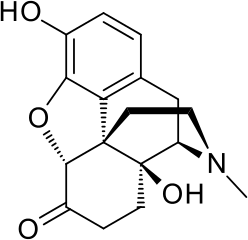



MJoules Rocket Shock Density Boil
/kg km/s km/s g/cm3 Kelvin
Beryllium+ O2 23.2 5.3
Aluminum + O2 15.5
Magnesium+ O2 14.8
Hydrogen + O2 13.2 4.56 .07 20
Kerosene + O3 12.9
Octanitrocubane 11.2 10.6 1.95
Methane + O2 11.1 3.80 .42 112 CH4
Octane + O2 10.4 .70 399 C8H18
Kerosene + O2 10.3 3.52 .80 410 C12H26
Dinitrodiazeno. 9.2 10.0 1.98
C6H6N12O12 9.1 1.96 China Lake compound
Kerosene + H2O2 8.1 3.2
Kerosene + N2O4 8.0 2.62
HMX (Octogen) 8.0 3.05 9.1 1.86
RDX (Hexagen) 7.5 2.5 8.7 1.78
Al + NH4NO3 6.9
Nitroglycerine 7.2 8.1 1.59 Unstable
PLX 6.5 1.14 95% CH3NO2 + 5% C2H4(NH2)2
Composition 4 6.3 8.04 1.59 91% RDX. "Plastic explosive"
Kerosene + N2O 6.18
Dynamite 5.9 7.2 1.48 75% Nitroglycerine + stabilizer
PETN 5.8 8.35 1.77
Smokeless powder 5.2 6.4 1.4 Used after 1884. Nitrocellulose
TNT 4.7 6.9 1.65 Trinitrotoluene
Al + Fe2O3 4.0 Thermite
H2O2 2.7 1.59 1.45 423 Hydrogen peroxide
Black powder 2.6 .6 1.65 Used before 1884
Al + NH4ClO4 2.6
NH4ClO4 2.5
N2O 1.86 1.76
N2H4 1.6 2.2 1.02 387 Hydrazine
NH4NO3 1.4 2.0 2.55 1.12 Ammonium nitrate
Bombardier beetle .4 Hydroquinone + H2O2 + protein catalyst
N2O4 .10 1.45 294
Rocket: Rocket exhaust speed
Shock: Shock speed
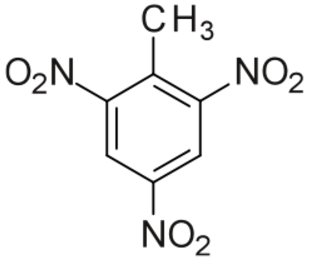
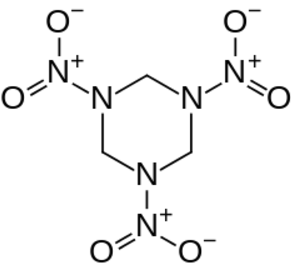
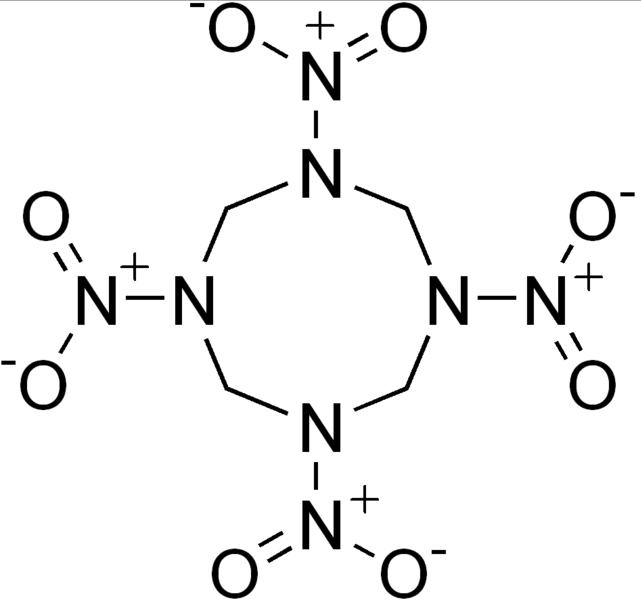
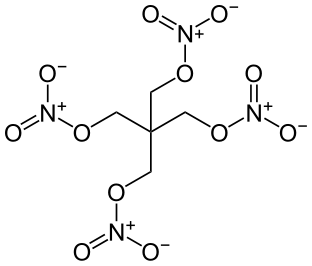
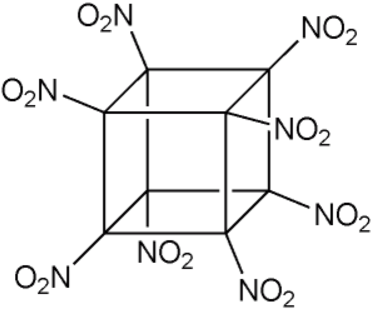
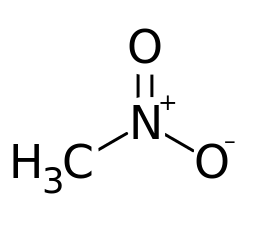
MJoules Shock Density
/kg km/s g/cm3
Octanitrocubane 11.2 10.6 1.95
Dinitrodiazeno. 9.2 10.0 1.98
C6H6N12O12 9.1 1.96 China Lake compound
HMX (Octogen) 8.0 9.1 1.86
RDX (Hexagen) 7.5 8.7 1.78
PLX 6.5 1.14 95% CH3NO2 + 5% C2H4(NH2)2
Composition 4 6.3 8.04 1.59 91% RDX. "Plastic explosive"
Dynamite 5.9 7.2 1.48 75% Nitroglycerine + stabilizer
PETN 5.8 8.35 1.77
Exhaust Energy Density of fuel + oxidizer
speed /mass
km/s MJ/kg g/cm3
Hydrogen H2 4.46 13.2 .32
Methane CH4 3.80 11.1 .83
Ethane C2H6 3.58 10.5 .9
Kerosene C12H26 3.52 10.3 1.03
Hydrazine N2H4 3.46 1.07
Liquid hydrogen is usually not used for the ground stage of rockets because of
its low density.
Energy/Mass Energy/Mass Rocket Rocket Boil Density
with kerosene as monopropellant with kerosene as monopropellant Kelvin g/cm3
MJoule/kg MJoule/kg km/s km/s
O3 12.9 2.97 161
O2 10.3 0 3.52 0 110 1.14
H2O2 8.1 2.7 3.2 1.6 423 1.45
N2O4 8.00 .10 2.62 294 1.44
N2O 6.18 1.86 1.76 185
N2H4 - 1.58 2.2 387 1.02
MJoules Rocket Density
/kg km/s g/cm3
C6H6N12O12 9.1 1.96 China Lake compound
HMX (Octogen) 8.0 3.05 1.86
RDX (Hexagen) 7.5 2.5 1.78
Al + NH4ClO4 2.6
NH4ClO4 2.5
NH3OHNO3 2.5 1.84 Hydrxyammonium nitrate
Al + NH4NO3 6.9
NH4NO3 1.4 2.0 1.12 Ammonium nitrate
~808 Qing Xuzi publishes a formula resembling gunpower, consisting of
6 parts sulfur, 6 parts saltpeter, and 1 part birthwort herb (for carbon).
~850 Incendiary property of gunpower discovered
1132 "Fire lances" used in the siege of De'an, China
1220 al-Rammah of Syria publishes "Military Horsemanship and Ingenious War
Devices", describes the purification of potassium nitrate by
adding potassium carbonate with boiling water, to precipitate out
magnesium carbonate and calcium carbonate.
1241 Mongols use firearms at the Battle of Mohi, Hungary
1338 Battle of Arnemuiden. First naval battle involving cannons.
1346 Cannons used in the Siege of Calais and the Battle of Crecy
1540 Biringuccio publishes "De la pirotechnia", giving recipes for gunpowder
1610 First flintlock rifle
1661 Boyle publishes "The Sceptical Chymist", a treatise on the
distinction between chemistry and alchemy. It contains some of the
earliest modern ideas of atoms, molecules, and chemical reaction,
and marks the beginning of the history of modern chemistry.
1669 Phosphorus discovered
1774 Lavoisier appointed to develop the French gunpowder program. By 1788
French gunpowder was the best in the world.
1832 Braconnot synthesizes the first nitrocellulose (guncotton)
1846 Nitrocellulose published
1847 Sobrero discovers nitroglycerine
1862 LeConte publishes simple recipes for producing potassium nitrate.
1865 Abel develops a safe synthesis of nitrocellulose
1867 Nobel develops dynamite, the first explosive more powerful than black powder
It uses diatomaceous earth to stabilize nitroglycerine
1884 Vieille invents smokeless gunpowder (nitrocellulose), which is 3 times
more powerful than black powder and less of a nuisance on the battlefield.
1902 TNT first used in the military. TNT is much safer than dynamite
1930 RDX appears in military applications
1942 Napalm developed
1949 Discovery that HMX can be synthesized from RDX
1956 C-4 explosive developed (based on RDX)
1999 Eaton and Zhang synthesize octanitrocubane and heptanitrocubane
Black powder = .75 KNO3 + .19 Carbon + .06 Sulfur







Potassium nitrate KNO3 75% (Saltpeter)
Charcoal C7H4O 15%
Sulfur S 10%
Oversimplified equation: 2 KNO3 + 3 C + S → K2S + N2 + 3 CO2
Realistic equation: 6 KNO3 + C7H4O + 2 S → KCO3 + K2SO4 + K2S + 4 CO2 + 2 CO + 2 H2O + 3 N2
Nitrite (NO3) is the oxidizer and sulfur lowers the ignition temperature.
MJoules
/kg
Hydrogen + Oxygen 13.16
Gasoline + Oxygen 10.4
Mass Energy Energy/Mass
kg MJ MJ/kg
MOAB 9800 46000 4.7 8500 kg of fuel



Form Ignition Density
(Celsius)
White 30 1.83
Red 240 1.88
Violet 300 2.36
Black 2.69
Red phosphorus is formed by heating white phosphorus to 250 Celsius or by
exposing it to sunlight. Violet phosphorus is formed by heating red phosphorus
to 550 Celsius. Black phosphorus is formed by heating white phosphorus at a
pressure of 12000 atmospheres. Black phosphorus is least reactive form and it
is stable below 550 Celsius.




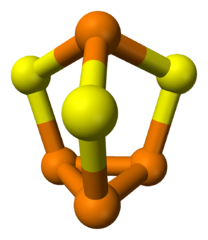
Match head Fraction Striking surface Fraction
Potassium chlorate KClO3 .50 Red phosphorus .5
Silicon filler Si .4 Abrasive .25
Sulfur S small Binder .16
Antimony3 trisulfide Sb2S3 small Neutralizer .05
Neutralizer small Carbon .04
Glue small
A "strike anywhere" match has phosphorus in the match head in the form of
phosphorus sesquisulfide (P4S3) and doesn't need red
phosphorus in the striking surface. P4S3 has an ignition
temperature of 100 Celsius.

.jpg)
Cerium .38 Ignition temperature of 165 Celsius
Lanthanum .22
Iron .19
Neodymium2 .04
Praseodymium .04
Magnesium .04
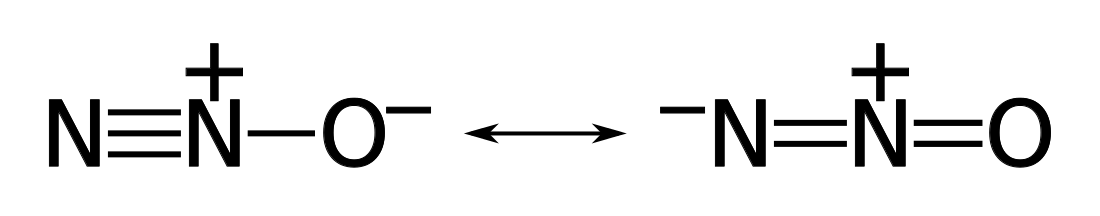
Air density = .00122 g/cm3
Nitrous oxide gas density = .00198 g/cm3
Diesel density = .832 g/cm3
Gasoline density = .745 g/cm3
Diesel energy/mass = 43.1 MJoules/kg
Gasoline energy/mass = 43.2 MJoules/kg
Nitrous oxide boiling point = -88.5 Celsius
Air oxygen fraction = .21
Nitrous oxide oxygen fraction= .33
Nitrous oxide decompose temp = 300 Celsius
Nitrous oxide liquid pressure= 52.4 Bars Pressure required to liquefy N2O at room temperature

2 H2O2 → 2 H2O + O2 (with protein catalyst)
C6H4(OH)2 → C6H4O2 + H2 (with protein catalyst)
O2 + 2 H2 → 2 H2O
Firing rate = 500 pulses/second
Number of pulses in one barrage = 70
Firing time = .14 seconds
Number of barrages = 20




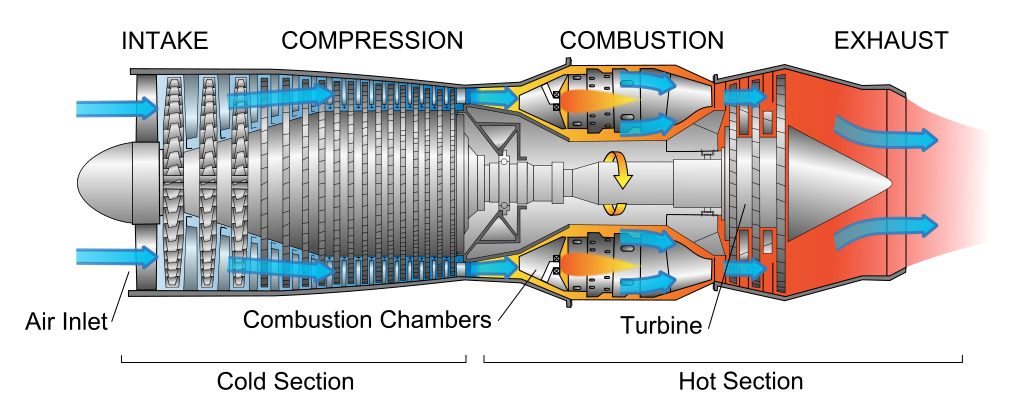
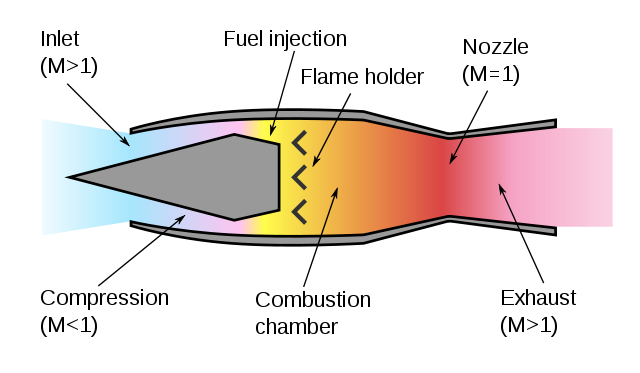
Airbus A350 compression ratio = 52
Air density at sea level = 1 bar
Air density at 15 km altitude = .25 bar
Air density in A350 engine = 13 bar
From the thermal flame theory of Mallard and Le Chatelier,
Temperature of burnt material = Tb
Temperature of unburnt material = Tu
Temperature of ignition = Ti
Fuel density = Dfuel
Oxygen density = Doxygen
Reaction coefficient = C
Reaction rate = R = C Dfuel Doxygen
Thermal diffusivity = Q = 1.9⋅10-5 m2/s
Flame speed = V
V2 = Q C Dfuel Doxygen (Tb - Ti) / (Ti - Tu)


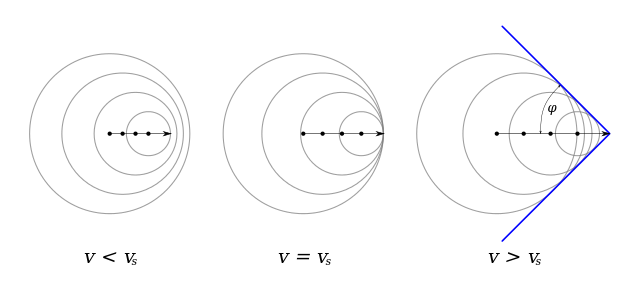
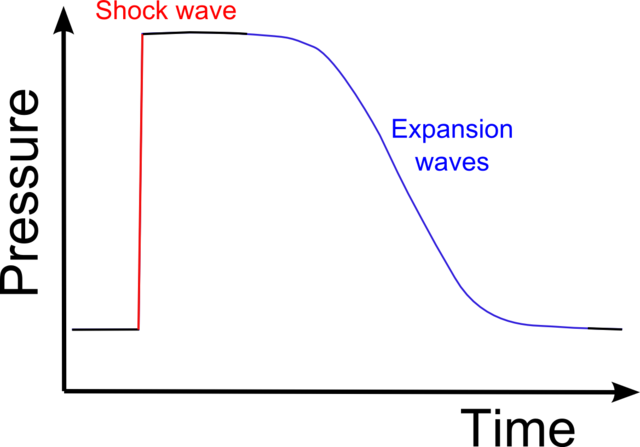
Energy/mass Energy/mass
not including including
oxygen oxygen
(MJoule/kg) (MJoule/kg)
Hydrogen 113.4 12.7
Gasoline 46.0 10.2
Beryllium 64.3 23.2
Aluminum 29.3 15.5
Magnesium 24.5 14.8
Carbon 12.0 3.3
Lithium 6.9 3.2
Iron 6.6 4.6
Copper 2.0 1.6



































Bullet Bullet Speed Energy Barrel Gun Fire Vehicle Cartridge
diam mass rate mass
mm kg m/s kJoule meters kg Hertz tons
Swiss Mini Gun 2.3 .00013 122 .00097 .0018 .020
Chiappa 17 4.4 .0010 560 .16 .121 .17 PMC/Aguila
Chiappa 17 4.4 .0010 640 .20 .121 .17 HM2
SPP-1 4.5 .0128 245 .38 .95 4.5x40mmR
Heckler Koch MP7 4.6 .0020 735 .54 .180 1.9 HK 4.6x30mm
Walther PPK 5.6 .0020 530 .281 .083 .560
Walther PPK 5.6 .0030 370 .141 .083 .560
Walther PPK/S 7.65 .0050 318 .240 .083 .630
Walther PPK/E 9.0 .0065 323 .338 .083 .665
Luger 9mm 9.0 .0081 354 .102
Winchester 9x23 9.0 .0081 442
Colt 45 11.4 .0100 262 .127
Magnum 44 11.2 .0156 448 .165
Smith Wesson 460 11.5 .019 630 3.77 .213 .460 SW Magnum
Magnum DesertEagle 12.7 .019 470 .254 1.996 .50 Action Express
Smith Wesson 50mag 12.7 .026 550 .267 2.26
Smith Wesson 50mag 12.7 .029 520 3.92 .267 2.26
Smith Wesson 50mag 12.7 .032 434 3.01 .267 2.26 .500 SW Magnum
MagnumResearch BFR 12.7 .026 550 3.93 .254 2.40 .50 Beowulf
Ruger 96 4.4 .0013 720 .34 .47 2.38 .17 HMR
Ruger M77 5.2 .0026 1200 1.83 .61 3.74 .204 Ruger
CMMG MK47 Mutant 5.6 .0036 975 Remmington 22
Remmington 9mm 9.0 .0091 975
M4 Carbine 5.56 .0041 936 1.80 .370 2.88 15.8
FN SCAR-H Rifle 7.62 .011 790 3.51 .400 3.58 10.4 20 round magazine
Barrett M82 13.0 .045 908 18.9 .74 14.0 10 round magazine
Hannibal 14.9 .049 750 13.8
CZ-550 15.2 .065 914 27.2 .600 Overkill
Vidhwansak 20 .13 720 33.7 1.0 26 20x81 mm. 3 round magazine
RT-20 20 .13 850 47 .92 19.2 1 round magazine
M621 cannon 20 .102 1005 51.5 45.5 13.3 20x102 mm
M61 Vulcan 20 .102 1050 56.2 92 110 20x102. 6 barrels
Oerlikon KBA 25 .184 1335 164 2.888 112 10
M242 Bushmaster 25 .184 1100 111 2.175 119 8.3 27.6 M2 Bradley
GAU-12 Equalizer 25 .184 1040 99.5 122 70 6.3 Harrier 2. 5 barrels
M230 chain gun 30 .395 805 128 55.9 10.4 5.2 Apache. 30x113 mm
Mk44 Bushmaster 2 30 .395 1080 230 2.41 160 3.3 27.6 M2 Bradley. 30x173 mm
GAU-8 Avenger 30 .395 1070 226 2.30 281 70 11.3 A-10 Warthog. 30x173 mm. 7 barrels
Bushmaster III 35 1180 218 3.3 35x228 mm
Bushmaster IV 40 1.08 198 3.3 40x365 mm
Rheinmetall 120 120 8.350 1750 12800 6.6 4500 .1 62 M1 Abrams tank
M777 Howitzer 155 48 827 16400 5.08 4200 .083
Iowa Battleship 406 862 820 290000 20.3 121500 .033 45000
2 bore rifle 33.7 .225 460 23.7 .711 4.5 Historical big-game rifle
Cannonball 6 lb 87 2.72 438 261 2.4
Cannonball 9 lb 96 4.08 440 395 2.7
Cannonball 12 lb 110 5.44 453 558 2.4
Cannonball 18 lb 125 8.16 524 1120 2.6 2060
Cannonball 24 lb 138 10.89 524 1495 3.0 2500
Cannonball 32 lb 152 14.5 518 1945 3.4 2540
Cannonball 36 lb 158 16.33 450 1653 2.9 3250
Cannonball diameters are calculated from the mass assuming a density of 7.9
g/cm3.
For a pistol or rifle, the "vehicle mass" is the mass of the person wielding it. We
use the mass of a typical person.
The "Metal Storm" gun has 36 barrels, 5 bullets per barrel, and fires all bullets
in .01 seconds. The bullets are stacked in the barrel end-to-end and fired sequentially.






Bullet energy .32
Hot gas .34
Barrel heat .30
Barrel friction .02
Unburnt powder .01
To estimate the velocity of a bullet,
Energy efficiency = e = .32 (Efficiency for converting powder energy to bullet enery)
Bullet mass = M
Powder mass = m
Powder energy/mass = Q = 5.2 MJoules/kg
Bullet velocity = V
Bullet energy = E = ½ M V2 = e Q m (Kinetic energy = Efficiency * Powder energy)
V = (2 e Q m / M)2 = 1820 (m/M)½ meters/second






















Color Colorant carat ($)
Painite 55000 CaZrAl9O15(BO3)
Diamond Clear 1400 C
Ruby Red Chromium 15000 Al2O3
Sapphire Blue Iron 650 Al2O3
Sapphire yellow Titanium Al2O3
Sapphire Orange Copper Al2O3
Sapphire Green Magnesium Al2O3
Emerald Green Chromium Be3Al2(SiO3)6
Beryl Aqua Iron Be3Al2(SiO3)6 AKA "aquamarine"
Morganite Orange Manganese 300 Be3Al2(SiO3)6
Topaz Topaz Al2SiO4(F,OH)2
Spinel Red Red MgAl2O4
Quartz Clear SiO2
Amethyst Purple Iron SiO2
Citrine Yellow SiO2
Zircon Red ZrSiO4
Garnet Orange [Mg,Fe,Mn]3Al2(SiO4)3 & Ca3[Cr,Al,Fe]2(SiO4)3
Garnet Blue 1500 [Mg,Fe,Mn]3Al2(SiO4)3 & Ca3[Cr,Al,Fe]2(SiO4)3
Opal SiO2·nH2O
Opal Black 11000 SiO2·nH2O
Jet Black Lignite
Peridot Green (Mg,Fe)2SiO4
Pearl White CaCO3
Jade Green NaAlSi2O6
Amber Orange Resin

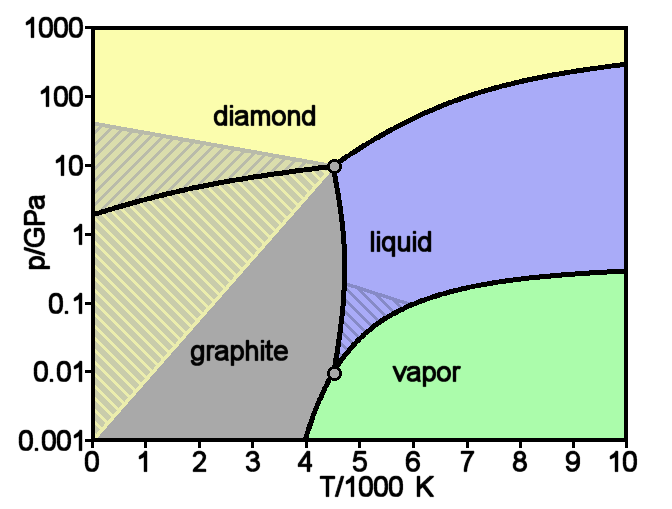
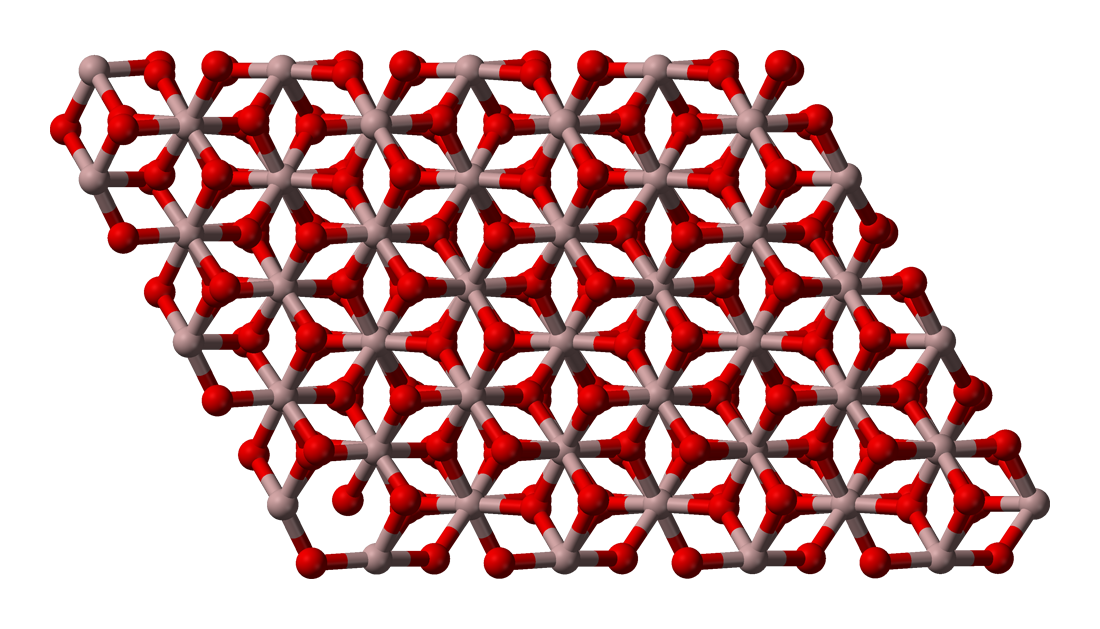
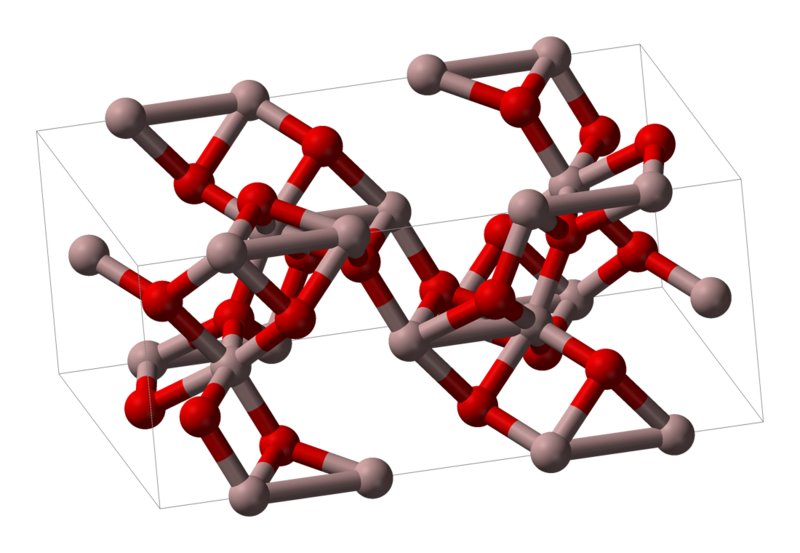
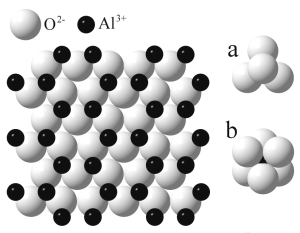
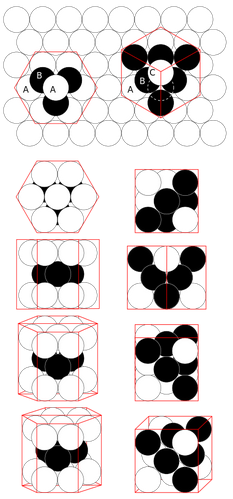
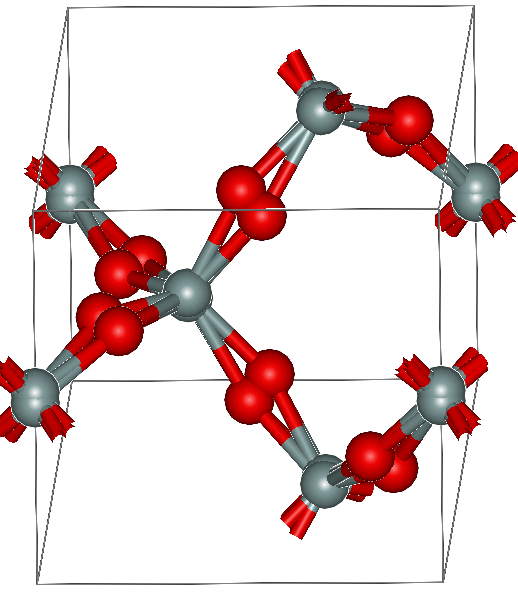



.gif)
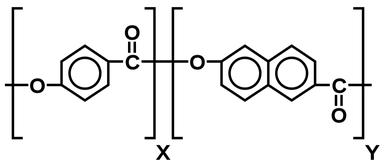
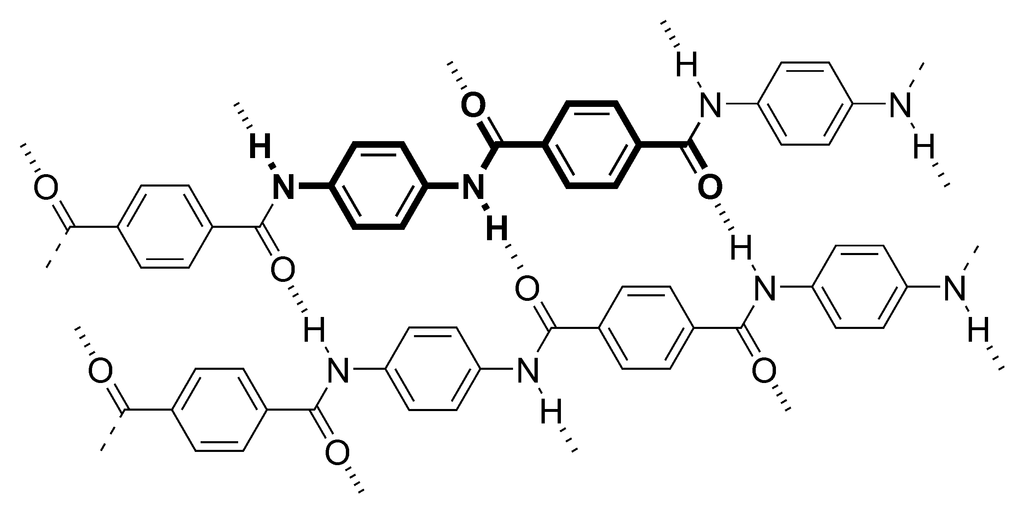
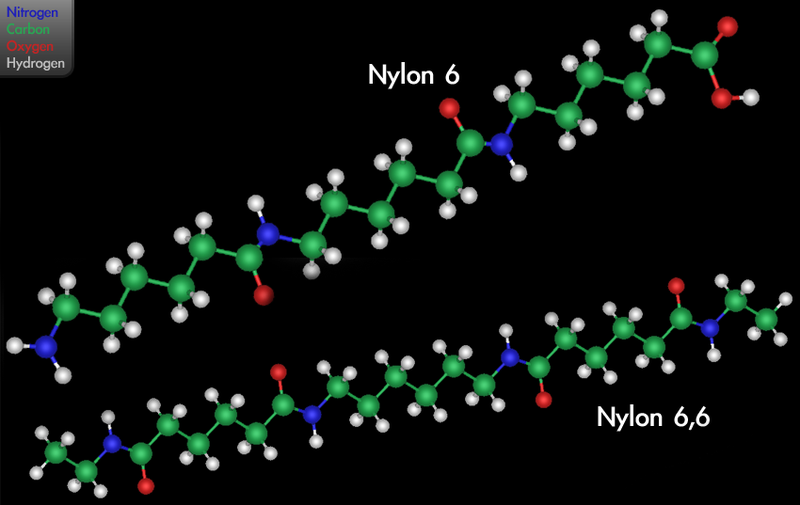
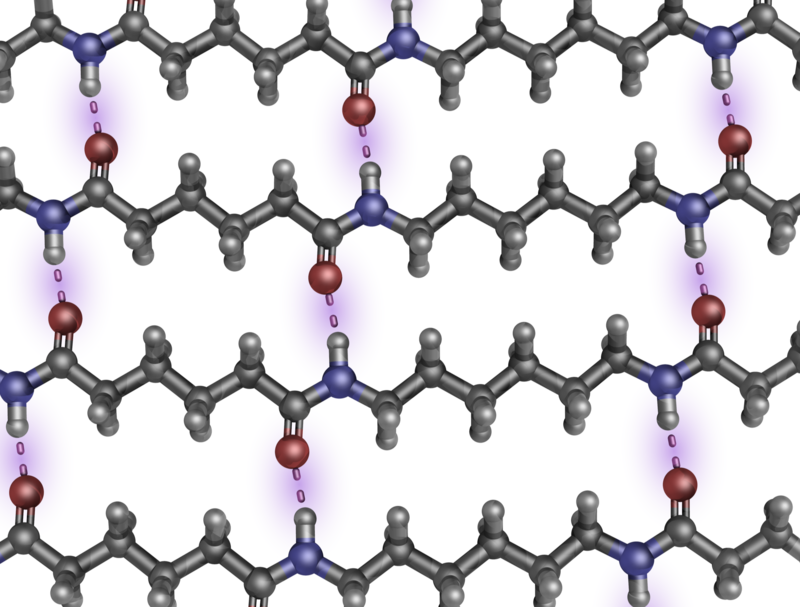
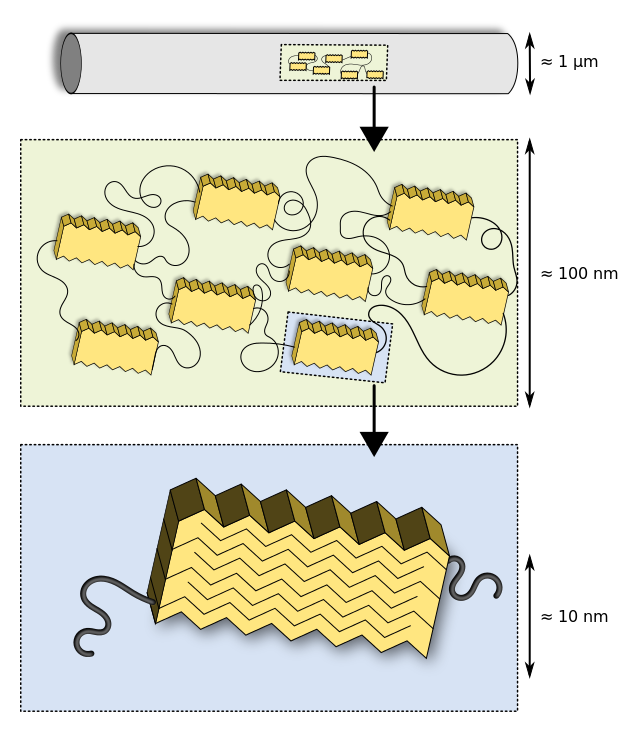
Year Young Tensile Strain Density Common
(GPa) strength (g/cm3) name
(GPa)
Gut Ancient .2
Cotton Ancient .1 1.5
Hemp Ancient 10 .3 .023
Duct tape .015
Gorilla tape .030
Polyamide 1939 5 1.0 .2 1.14 Nylon, Perlon
Polyethylene 1939 117 1.4 Dacron
Polyester 1941 15 1.0 .067 1.38
Polypropylene 1957 .91
Carbon fiber 1968 3.0 1.75
Aramid 1973 135 3.0 .022 1.43 Kevlar
HMPE 1975 100 2.4 .024 .97 Dyneema, Spectra
PBO 1985 280 5.8 .021 1.52 Zylon
LCAP 1990 65 3.8 .058 1.4 Vectran
Vectran HT 75 3.2 .043 1.41 Vectran
Vectran NT 52 1.1 .021 1.41 Vectran
Vectran UM 103 3.0 .029 1.41 Vectran
Nanorope ~1000 3.6 .0036 1.3
Nanotube 1000 63 .063 1.34
Graphene 1050 160 .152 1.0
Strain = Strength / Young
Carbon fiber is not useful as a rope.
Batman mass = M = 100 kg (includes suit and gear)
Gravity constant = g = 10 meters/second2
Batman weight = F = 1000 Newtons
Zylon density = D = 1520 kg/meter3
Zylon tensile strength = Pz = 5.8⋅109 Newtons/meter2
Rope load = P = 1.0⋅109 Newtons/meter2 (safety margin)
Rope length = L 100 meters
Rope cross section = A = F/P =1.0⋅10-6 meters2
Rope radius = R =(A/π)½= .56 mm
Rope mass = Mr = DAL = .15 kg
Density Tensile Young
strength
(g/cm^3) (Gpa) (Gpa)
Balsa .12 .020 3.7
Corkwood .21
Cedar .32 .046 5.7 Northern white
Poplar .33 .048 7.2 Balsam
Cedar .34 .054 8.2 Western red
Pine .37 .063 9.0 Eastern white
Buckeye .38 .054 8.3 Yellow
Butternut .40 .057 8.3
Basswood .40 .061 10.3
Spruce, red .41 .072 10.7
Aspen .41 .064 10.0
Fir, silver .42 .067 10.8
Hemlock .43 .061 8.5 Eastern
Redwood .44 .076 9.6
Ash, black .53 .090 11.3
Birch, gray .55 .069 8.0
Walnut, black .56 .104 11.8
Ash, green .61 .100 11.7
Ash, white .64 .110 12.5
Oak, red .66 .100 12.7
Elm, rock .66 .106 10.9
Beech .66 .102 11.8
Birch, yellow .67 .119
Mahogany .67 .124 10.8 West Africa
Locust .71 .136 14.5 Black or Yellow
Persimmon .78 .127 14.4
Oak, swamp .79 .124 14.5 Swamp white
Gum, blue .80 .118 16.8
Hickory .81 .144 15.2 Shagbark
Eucalyptus .83 .122 18.8
Bamboo .85 .169 20.0
Oak, live .98 .130 13.8
Ironwood 1.1 .181 21.0
Lignum Vitae 1.26 .127 14.1
Data #1
Data #2
Density Tensile Young
strength
(g/cm^3) (Gpa) (Gpa)
Polyamide .11 4.5
Polyimide .085 2.5
Acrylic .07 3.2
Polycarbonate .07 2.6
Acetyl copoly .06 2.7
ABS .04 2.3
Polypropylene .91 .04 1.9
Polystyrene .04 3.0
Polyethylene .95 .015 .8


Yield Density Strength/Density
strength (g/cm3) (GJoule/kg)
(GPascal)
Beryllium .34 1.85 .186
Aluminum + Be .41 2.27 .181
LiMgAlScTi 1.97 2.67 .738
Titanium .22 4.51 .050
Titanium + AlVCrMo 1.20 4.6 .261
Vanadium .53 6.0 .076
AlCrFeCoNiTi 2.26 6.5 .377
AlCrFeCoNiMo 2.76 7.1 .394
Steel .25 7.9 .032 Iron plus carbon
Copper .12 9.0 .013
"Yield strength" is the maximum pressure a material can sustain before
deforming. "Strength/Density" is the strength-to-weight ratio.
Steel is stronger and lighter than copper.
Name Owner
Sword Longclaw Jon Snow
Sword Heartsbane Samwell Tarly
Dagger Arya
Sword Ice Eddard Stark Reforged into Oathkeeper and Widow's Wail
Sword Oathkeeper Brienne of Tarth
Sword Widow's Wail The Crown
Sword Lady Forlorn Ser Lyn Corbray
Sword Nightfall Ser Harras Harlow
Sword Red Rain Lord Dunstan Drumm
Arakh Caggo
Armor Euron Greyjoy
Horn Dragonbinder The Citadel of The Maesters
Some Maesters carry links of Valyrian steel, a symbol of mastery of the highest arts.


C8H18 + 12.5 O2 → 8 CO2 + 9 H2O
3 C + S + 2 KNO3 → K2S + N2 + 3 CO2
We know that wildfire contains an oxidizer otherwise it wouldn't be able to
explode as it did on the show. Wildfire is made from manure, which contains
KNO3.
Gasoline: Flame spreads slowly. Needs oxygen from the air.
Gunpowder: Contains oxygen. Buns faster than gasoline. Subsonic pressure wave.
Plastic explosive: Pressure wave spreads supersonically as a shock.
~808 Qing Xuzi publishes a formula resembling gunpower, consisting of
6 parts sulfur, 6 parts saltpeter, and 1 part birthwort herb (for carbon).
~850 Incendiary property of gunpower discovered
1540 Biringuccio publishes "De la pirotechnia", giving recipes for gunpowder
1661 Boyle publishes "The Sceptical Chymist", a treatise on the
distinction between chemistry and alchemy. It contains some of the
earliest modern ideas of atoms, molecules, and chemical reaction,
and marks the beginning of the history of modern chemistry.
1662 Boyle discovers that for air at fixed temperature,
Pressure * Volume = Constant
1663 Guericke invents the first electrostatic generator, which uses
mechanical work to separate charge. Generators were refined until
they were superceded by the battery.
1671 Boyle discovers that combining iron filings and acid produces hydrogen gas.
1754 Black isolates CO2
1758 Black formulates the concept of latent heat to explain phase transitions
1766 Cavendish identifies hydrogen as a colorless, odourless gas that burns
in air.
1772 Scheele produces pure oxygen gas by heating HgO.
1774 Priestly produces pure oxygen gas by focusing sunlight on HgO.
He noted that it is combustible and that it gives energy when breathed.
1745 von Kleist invents the capacitor, a device for storing charge generated
by an electrostatic generator.
1746 van Musschenbroek refines the capacitor, which comes to be known as a
"Leyden jar".
1777 Lavoisier finds that in the reaction tin+oxygen, mass is conserved.
He also finds that oxygen is not the only component of air, that air also
consists of something else.
1780 Galvani observes that when a frog leg is touched by an iron scalpel,
it twitches. This was the inspiration for Volta to invent the battery.
1781 Cavendish finds that buring hydrogen + oxygen produces water.
1787 Charles finds that for air at constant pressure,
Volume = Constant * Temperature
He also finds that this applies for O2, N2, H2, and CO2.
1789 Lavoisier publishes "Traite Elementaire de Chimie", the first modern
chemistry textbook. It is a complete survey of (at that time) modern
chemistry, the law of conservation of mass.
1791 Volta develops the first electrochemical cell, consisting of two different
metals separated by a moist intermediary.
1797 Proust proposes the law of definite proportions, that elements
combine in small whole number ratios to form compounds.
1800 Volta constructs the first "battery" by connecting multiple electrochemical
cells in parallel, increasing the output power and voltage.
1801 Dalton publishes the law of partial pressures.
The pressure of a mix of gases is equal to the sum of the pressures
of the components. He also finds that when a light and heavy gas are mixed,
the heavy gas does not drift to the bottom but rather fills the space
uniformly.
1805 Gay-Lussac and Humboldt find that water is formed of two volumes of
hydrogen gas and one volume of oxygen gas.
1809 Gay-Lussac finds that for an ideal gas at constant volume,
Pressure = Constant * Temperature
1811 Avogadro finds that equal volumes of different gases have the same number
of particles. At constant temperature and pressure,
Volume = Constant * NumberOfParticles
1811 Avogadro arrives at the correct interpretation of water's composition,
based on what is now called Avogadro's law and the assumption of diatomic
elemental molecules
1840 Hess finds that energy is conserved in chemical reactions
1848 Lord Kelvin establishes concept of absolute zero, the temperature at
which all molecular motion ceases.
1860 Cannizzaro publishes a table of atomic weights of the known elements
1864 Gulberg and Waage propose the law of mass action
1869 Mendeleev publishes a periodic table containing the 66 known elements
1876 Gibbs publishes the concept of "Gibbs free energy"
1877 Boltzmann defines entropy and develops thermodynamics
1877 Pictet freezes CO2 and liquefies oxygen.
Liquification enables the purification of gases.
1894 Ramsay discovers the noble gases, filling a large and unexpected gap in
the periodic table
1635 Gassendi measures the speed of sound to be 478 m/s with 25% error.
1660 Viviani and Borelli produce the first accurate measurement of the speed of
sound, giving a value of 350 m/s.
1660 Hooke's law published. The force on a spring is proportional to the change
in length.
1662 Boyle discovers that for air at fixed temperature,
Pressure * Volume = Constant
Hence, air obey's Hooke's law
1687 Newton publishes the Principia Mathematica, which contains the first analytic
calculation of the speed of sound. The calculated value was 290 m/s
and the true value is 342 m/s (at 20 Celsius).
Newton's result was the first solid evidence for the existence of atoms. His
result differed from the correct value because it had not yet been discovered
that air heats when compressed. If you add this effect you get the right
value.
1789 Lavoisier publishes "Traite Elementaire de Chimie", the first modern
chemistry textbook. It is a complete survey of (at that time) modern
chemistry, the law of conservation of mass.
1797 Proust proposes the law of definite proportions, that elements
combine in small whole number ratios to form compounds.
1801 Dalton publishes the law of partial pressures.
The pressure of a mix of gases is equal to the sum of the pressures
of the components. He also finds that when a light and heavy gas are mixed,
the heavy gas does not drift to the bottom but rather fills the space
uniformly.
1805 Gay-Lussac and Humboldt find that water is formed of two volumes of
hydrogen gas and one volume of oxygen gas.
1809 Gay-Lussac finds that for an ideal gas at constant volume,
Pressure = Constant * Temperature
1811 Avogadro finds that equal volumes of different gases have the same number
of particles. At constant temperature and pressure,
Volume = Constant * NumberOfParticles
1811 Avogadro arrives at the correct interpretation of water's composition,
based on what is now called Avogadro's law and the assumption of diatomic
elemental molecules
1860 Cannizzaro publishes a table of atomic weights of the known elements
1869 Mendeleev publishes a periodic table containing the 66 known elements
1877 Boltzmann defines entropy and develops thermodynamics
All of these results support the hypothesis that matter is composed of atoms,
but there was no known experiment sensitive enough to measure the size and mass
of an individual atom.
Wavelength of violet light = 4e-7 meters
Diameter of an iron atom = 2e-10 meters
Violet photons are much larger than atoms and so you can't see atoms in an optical
microscope.
1905 Einstein publishes a method for measuring the mass of an atom using
Brownian motion
1908 Perrin uses Einstein's method to produce the first measurement of the mass of
an atom. This is equivalent to measuring the value of Avogadro's number.
Minutephysics atoms
© Jason Maron, all rights reserved.