The "de Broglie wavelength" of a particle is
Particle momentum = Q
Planck constant = h = 6.62*10^-34 Joule seconds
Particle wavelength = W = h/Q (de Broglie formula)
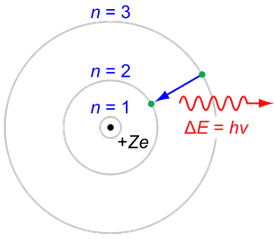
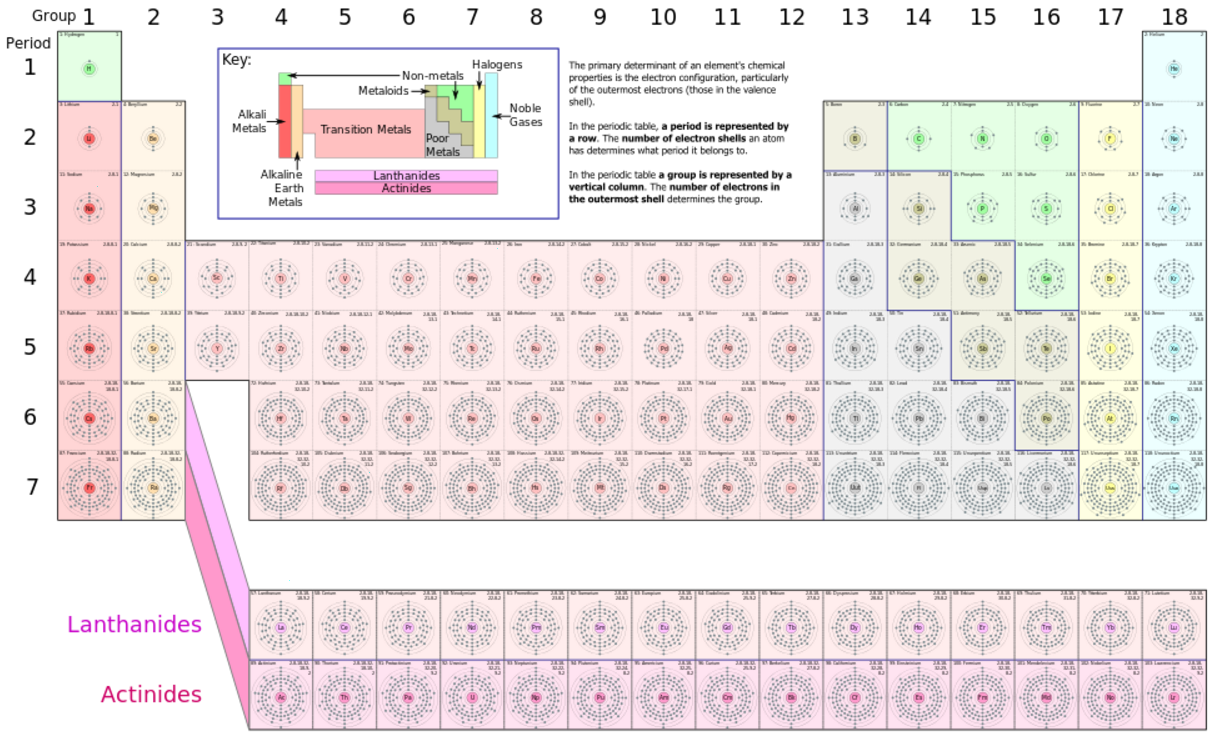
The Bohr hypothesis states that for an electron orbiting a proton, the number
of electron wavelengths is an integer. This sets the characteristic size of
a hydrogen atom.
Orbit circumference = C = N W where N is a positive integer
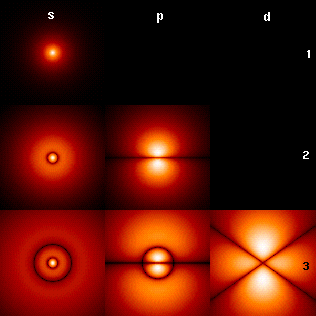
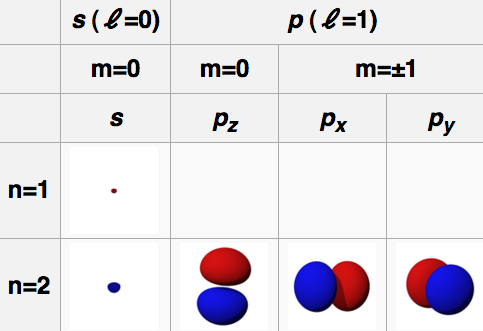
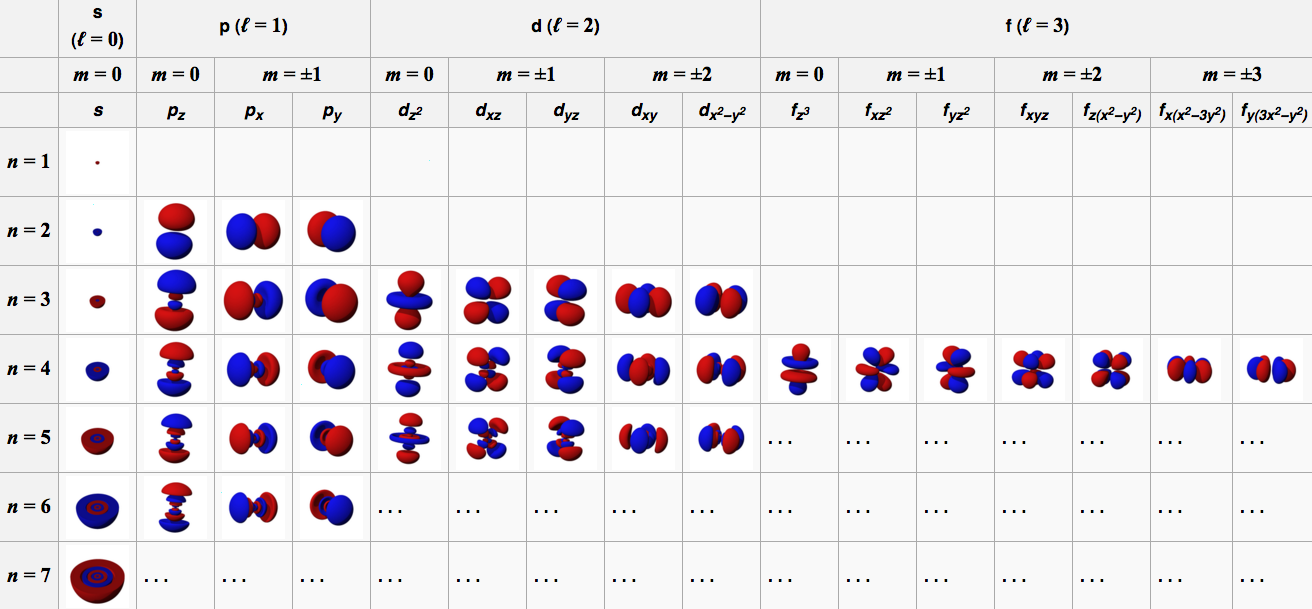
N Orbital
1 S
2 P
3 D
4 F
Electron mass = m = 9.11*10-31 kg
Electron velocity = V
Electron momentum = Q = m V
Electron charge = e = -1.60*10-19 Coulombs
Coulomb constant = K = 9.0*109 Newtons meters / Coulombs2
Electric force = Fe = K e2 / R2
Centripetal force = Fc = M V2 / R
Orbit radius = R = N h2 / (4 π2 K e2 m) = N * 5.29e-11 meters
Electron energy = E = - .5 K e2 / (R N2) = N-2 2.18e-18 Joules = N-2 13.6 electron Volts (Ionization energy)
For an electron on a circular orbit,
Fe = Fc