




 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
|---|
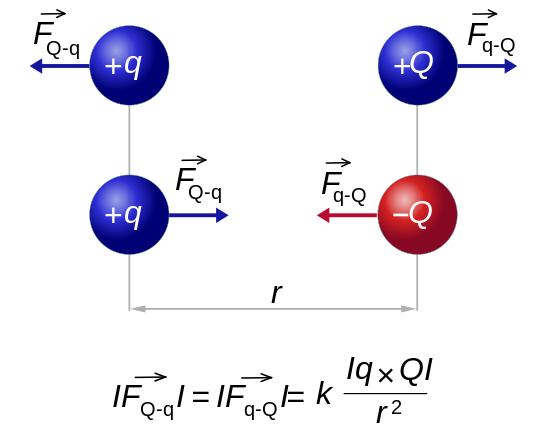
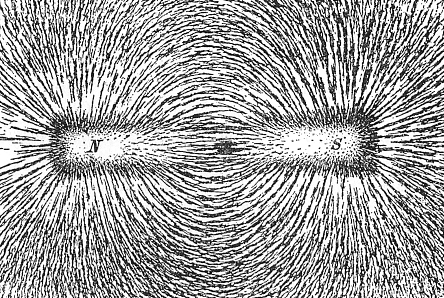
Charges of the same sign repel and charges of opposite sign attract.
Charge 1 Charge 2 Electric Force + + Repel - - Repel + - Attract - + Attract Charge = Q (Coulombs) 1 Proton = 1.602e-19 Coulombs Distance between charges = R Mass of the charges = M Gravity constant = G = 6.67e-11 Newton m2 / kg2 Electric constant = K = 8.99e9 Newton m2 / Coulomb2 Gravity force = F = -G M1 M2 / R2 = M2 g Electric force = F = -K Q1 Q2 / R2 = Q2 E Gravity field from M1 = g = G M1 / R2 Electric field from Q1 = E = K Q1 / R2 Gravity voltage = H g (H = Height, g = Gravitational acceleration) Electric voltage = H E (H = Distance parallel to the electric field) Gravity energy = -G M1 M2 / R Electric energy = -K Q1 Q2 / R
 |
|---|
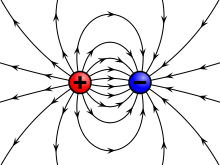
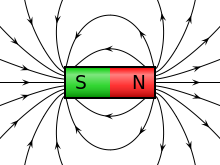
A charge generates an electric field. The electric field points away from positive charges and toward negative charges.
 |
 |
|---|---|
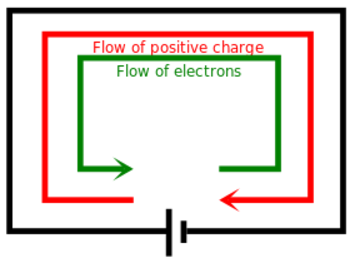
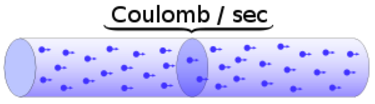
A moving charge is an "electric current". In an electric circuit, a battery moves electrons through a wire.
Charge = Q Time = T Electric current = I = Q / T (Coulombs/second)The current from a positive charge moving to the right is equivalent to that from a negative charge moving to the left.
 |
|---|
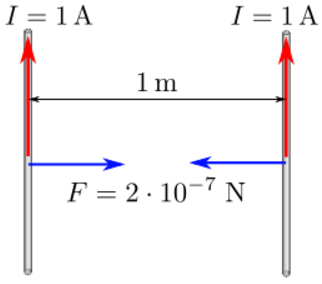
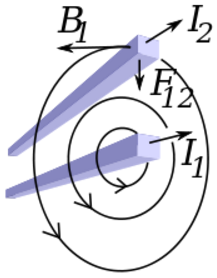
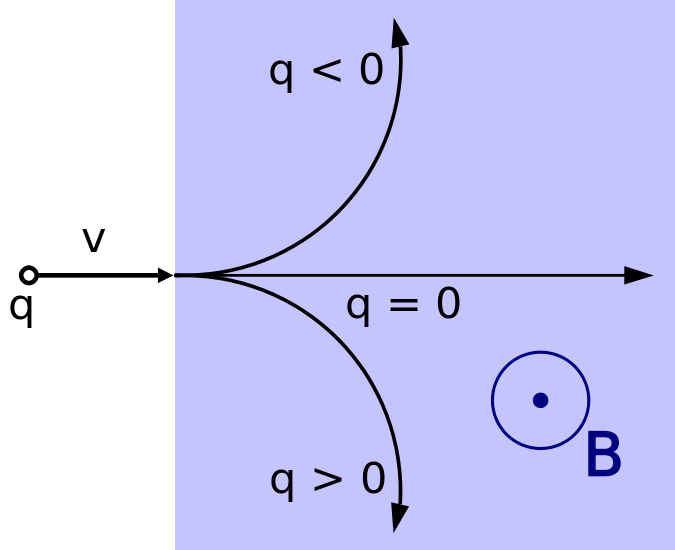
Moving charges and currents exert forces on each other. Parallel currents attract and antiparallel currents repel.
Charge = Q Velocity of the charges = V Current = I Length of a wire = L Distance between the charges = R Electric force constant = Ke = 8.988e9 N m2/C2 Magnetic force constant = Km = 2e-7 = Ke/C2 Electric force between charges = Fe = Ke Q1 Q2 / R2 Magnetic force between charges = Fm = Km V2 Q1 Q2 / R2 = (V2/C2) Fe Magnetic force between currents = Fm = Km I1 I2 Z / R Magnetic force / Electric force = V2 / C2The magnetic force is always less than the electric force.
 |
 |
|---|---|
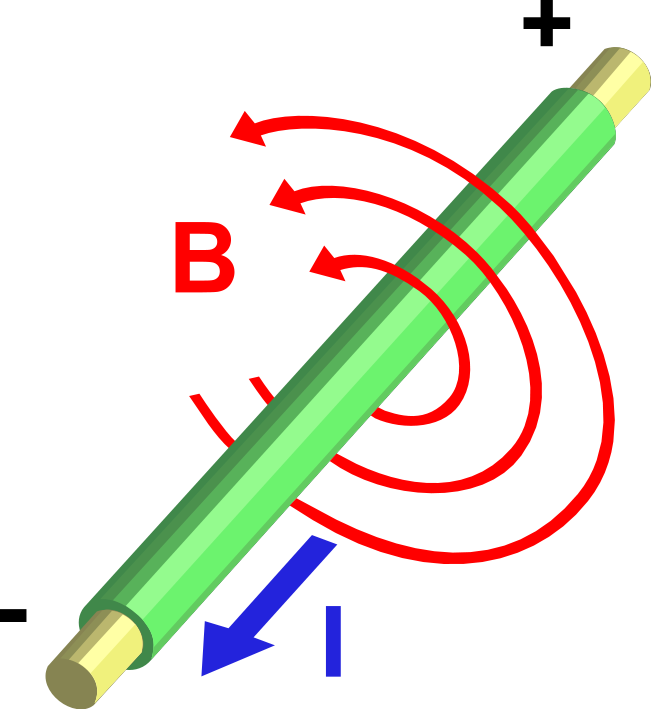
The electric force can be interpreted as an electric field, and the magnetic force can be interpreted as a magnetic field. Both interpretations produce the same force.
Radial distance = R (Distance perpendicular to the velocity of the charge) Magnetic field from charge Q1 = B = Km V Q1 / R2 Magnetic field from current I1 = B = Km I1 / R Magnetic force on charge Q2 = Fm = Q2 V B = Km V2 Q1 Q2 / R2 Magnetic force on current I2 = Fm = I2 Z B = Km I1 I2 Z / R
 |
 |
|
|---|---|---|
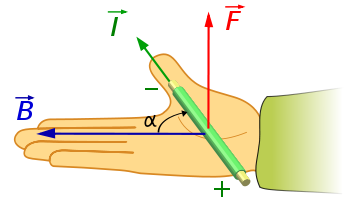
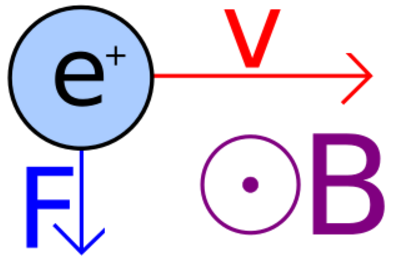
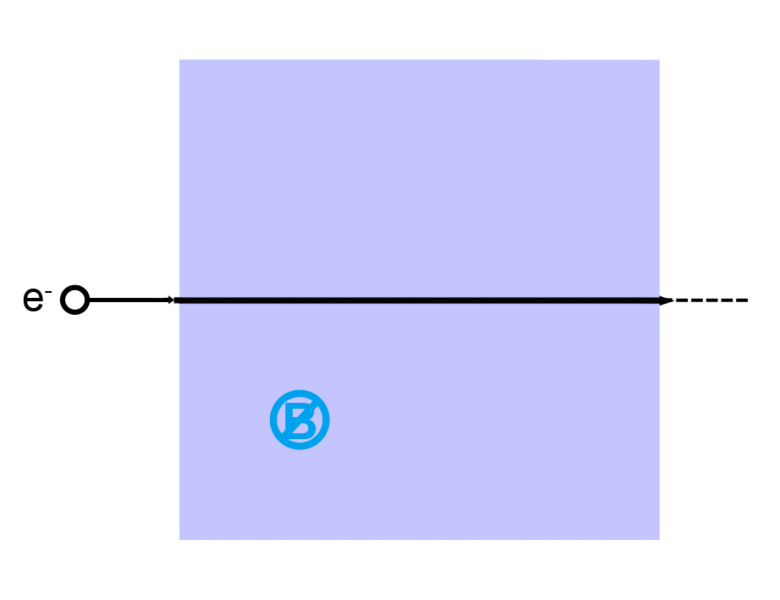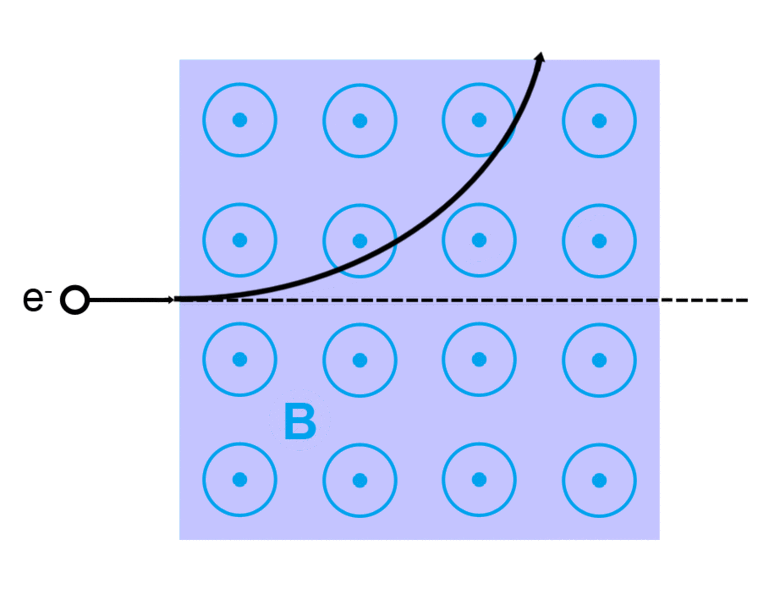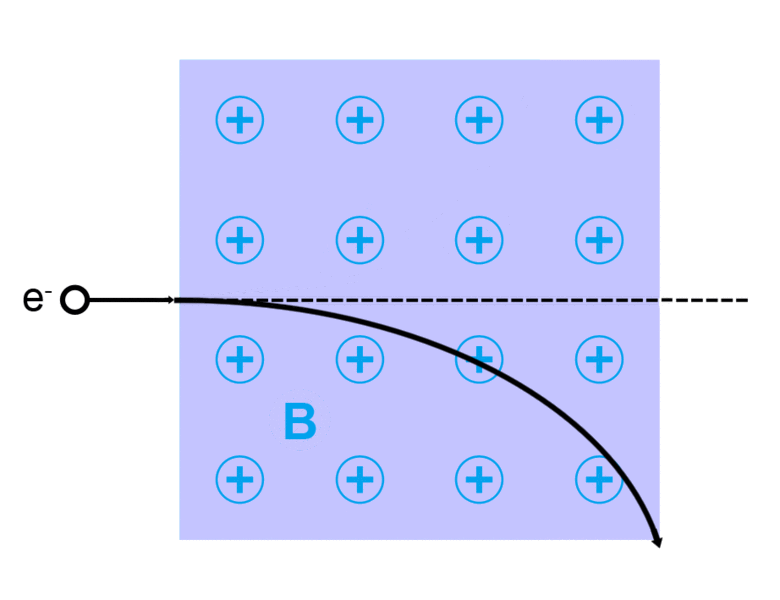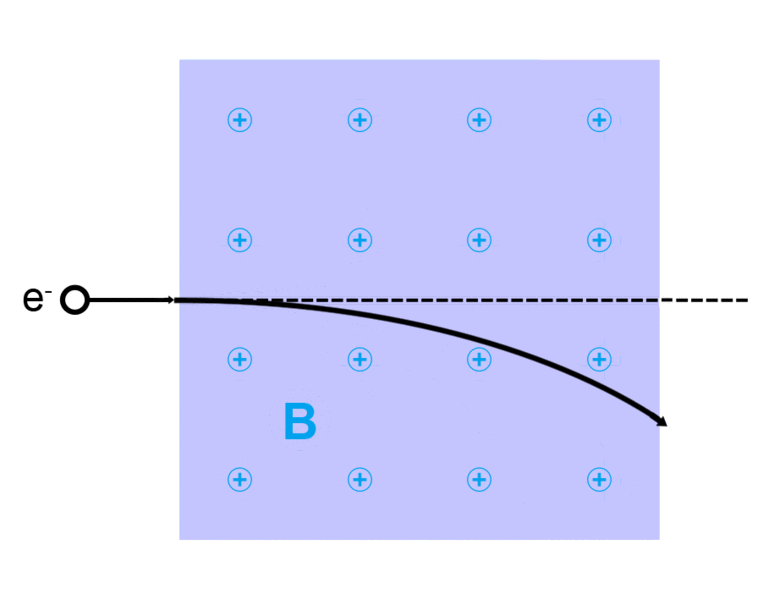
The direction of the magnetic force on a positive charge is given by the right hand rule. The force on a negative charge is in the opposite direction (the left hand rule).
 |
 |
 |
 |
|---|---|---|---|
 |
|---|
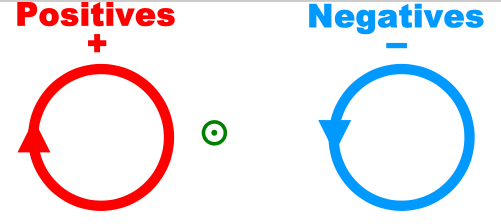
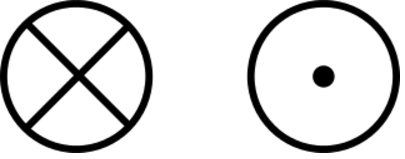
We use the above symbols to depict vectors in the Z direction. The vector on the left points into the plane and the vector on the right points out of the plane.
 |
 |
 |
|---|---|---|
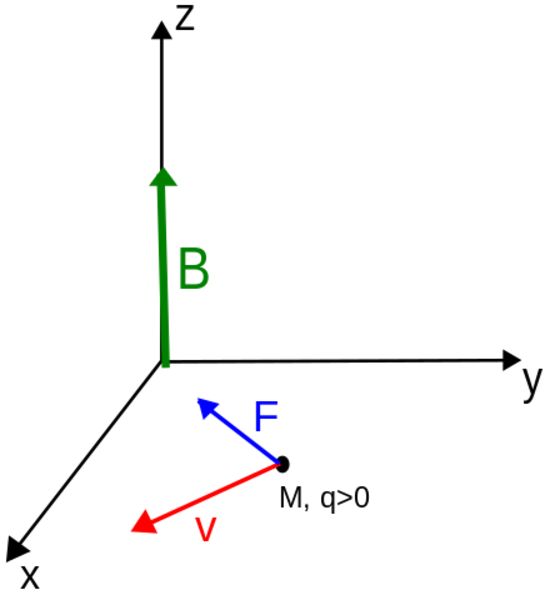
The direction of the force is the cross product "×" of V and B. The direction is given by the "right hand rule".
Magnetic field = B Magnetic force on a charge = F = Q V × B Magnetic force on a current = F = 2e-7 I × B
Quantity MKS units CGS units Conversion factor Mass M kg gram .001 Wire length Z meter cm .01 Radial distance from wire R meter cm .01 Time T second second 1 Force F Newton dyne 100000 Charge Q Coulomb Franklin 3.336e-10 Velocity of a charge V meter/second cm/s .01 Speed of light C 2.999e8 meter/second cm/s 100 Energy E Joule erg e-7 Electric current I Ampere = Coulomb/s Franklin/s 3.336e-10 Electric potential V Volt Statvolt 299.79 Electric field E Volt/meter StatVolt/cm 29979 Magnetic field B Tesla Gauss 10000 Capacitance C Farad cm 1.11e-12 Inductance L Henry s2/cm 9e-11 Electric force constant Ke = 8.988e9 N m2/C2 Ke = 1 dyne cm2 / Franklin2 Magnetic force constant Km = 2e-7 = Ke/C2 Km = 1/C2 Vacuum permittivity ε = 8.854e-12 F/m =1/4/π/Ke Vacuum permeability μ = 4 π e-7 Vs/A/m =2 π Km Proton charge Qpro = 1.602e-19 Coulomb Qpro= 4.803e-10 Franklin Electric field from a charge E = Ke Q / R2 E = Q / R2 Electric force on a charge F = Q E F = Q E Electric force between charges F = Ke Q Q / R2 F = Q Q / R2 Magnetic field of moving charge B = Km V Q / R2 B = (V/C) Q / R2 Magnetic field around a wire B = Km I / R B = (V/C) I / R Magnetic force on a charge F = Q V B F = (V/C) Q B Magnetic force on a wire F = Km B Z F = I B z Magnetic force between charges F = Km V2 Q1 Q2 / R2 F = (V/C)2 Q Q / R2 Magnetic force between wires F = Km I1 I2 Z / R F = I1 I2 Z / R Energy of a capacitor E = .5 C V2 Field energy per volume Z = (8 π Ke)-1 (E2 + B2/C2) Z = .5 (E2 + B2/C2)
Speed of light C Electric field E Electric field, time derivative Et Magnetic field B Magnetic field, time derivative Bt Charge Q Charge density q Current density J MKS CGS Ke=8.988e9 Ke=1 Km=2e-7 Km=2/C ∇˙E = 4 π Ke q ∇˙E = 4 π q ∇˙B = 0 ∇˙B = 0 ∇×E = -Bt ∇×E = -Bt / C ∇×B = 2 π Km J + Et / C2 ∇×B = 4 π J / C + Et / C
 |
 |
|---|---|
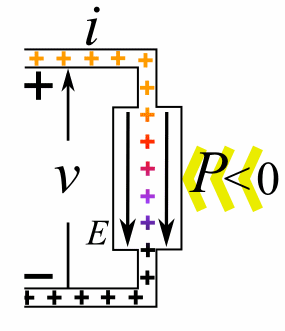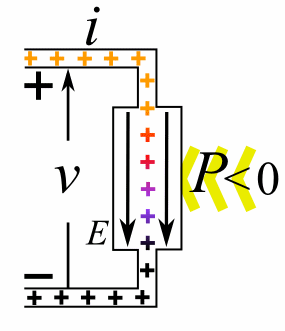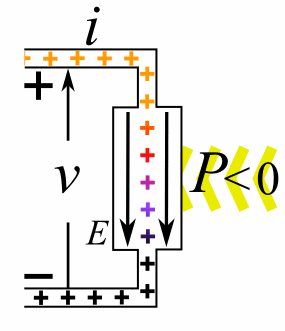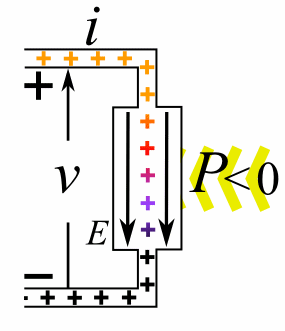
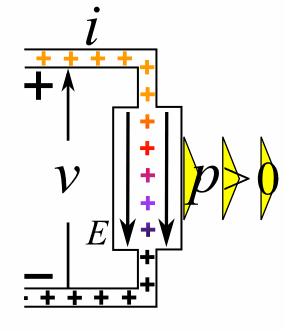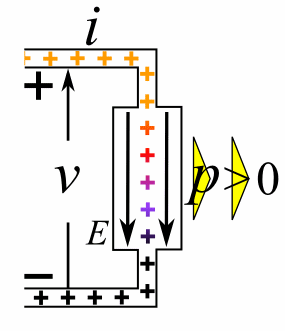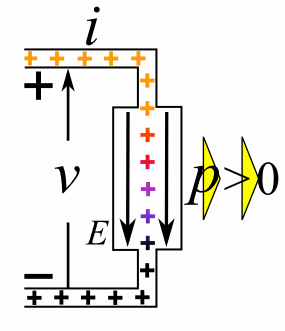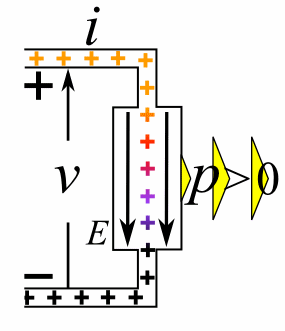
Charge = Q Coulombs
Voltage = V Volts
Energy = E = VQ Joules
Time = T seconds
Current = I = Q/T Amperes
Resistance = R = V/I Ohms
Power = P = QV/T Watts
= IV
= V2/R
= I2R
Ohm's Law: V = IR
 |
 |
|---|---|
In a superconductor, electrons move without interference.
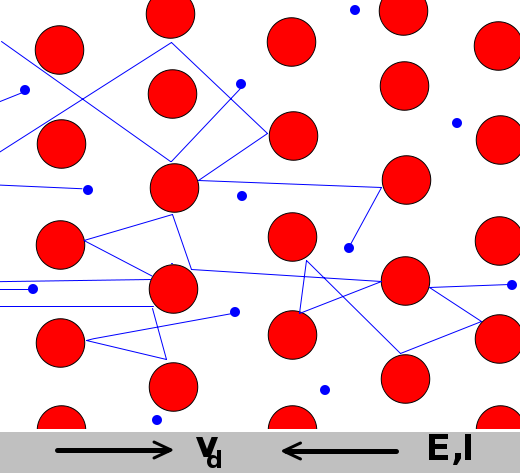
In a resistor, electrons collide with atoms and lose energy.
Resistance (Ohms)
Copper wire .02 1 meter long and 1 mm in diameter
1 km power line .03
AA battery .1 Internal resistance
Light bulb 200
Human 10000
 |
|---|
Voltage = V Volts Capacitance = C Farads Total energy = E = ½ C V2 Joules Effective = Ee = ¼ C V2 JoulesNot all of the energy in a capacitor is harnessable because the voltage diminishes as the charge diminishes, hence the effective energy is less than the total energy.
 |
|---|
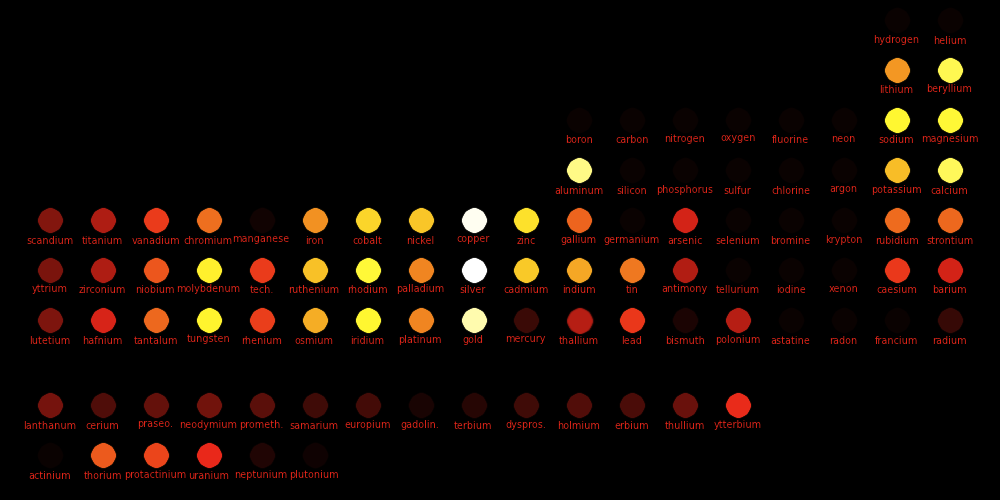
White: High conductivity Red: Low conductivity
Electric Thermal Density Electric C/Ct Heat Heat Melt $/kg Young Tensile Poisson Brinell
conduct conduct conduct/ cap cap number hardness
(e7 A/V/m) (W/K/m) (g/cm^3) Density (AK/VW) (J/g/K) (J/cm^3K) (K) (GPa) (GPa) (GPa)
Silver 6.30 429 10.49 .60 147 .235 2.47 1235 590 83 .17 .37 .024
Copper 5.96 401 8.96 .67 147 .385 3.21 1358 6 130 .21 .34 .87
Gold 4.52 318 19.30 .234 142 .129 2.49 1337 24000 78 .124 .44 .24
Aluminum 3.50 237 2.70 1.30 148 .897 2.42 933 2 70 .05 .35 .245
Beryllium 2.5 200 1.85 1.35 125 1.825 3.38 1560 850 287 .448 .032 .6
Magnesium 2.3 156 1.74 1.32 147 1.023 1.78 923 3 45 .22 .29 .26
Iridium 2.12 147 22.56 .094 144 .131 2.96 2917 13000 528 1.32 .26 1.67
Rhodium 2.0 150 12.41 .161 133 .243 3.02 2237 13000 275 .95 .26 1.1
Tungsten 1.89 173 19.25 .098 137 .132 2.54 3695 50 441 1.51 .28 2.57
Molybdenum 1.87 138 10.28 .182 136 .251 2896 24 330 .55 .31 1.5
Cobalt 1.7 100 8.90 .170 .421 1768 30 209 .76 .31 .7
Zinc 1.69 116 7.14 .388 693 2 108 .2 .25 .41
Nickel 1.4 90.9 8.91 .444 1728 15
Ruthenium 1.25 117 12.45 2607 5600
Cadmium 1.25 96.6 8.65 594 2 50 .078 .30 .20
Osmium 1.23 87.6 22.59 .130 3306 12000
Indium 1.19 81.8 7.31 430 750 11 .004 .45 .009
Iron 1.0 80.4 7.87 .449 1811 211 .35 .29 .49
Palladium .95 71.8 1828
Tin .83 66.8 505 22 47 .20 .36 .005
Chromium .79 93.9 .449 2180
Platinum .95 .133 2041
Tantalum .76 .140 3290
Gallium .74 303
Thorium .68
Niobium .55 53.7 2750
Rhenium .52 .137 3459
Vanadium .5 30.7 2183
Uranium .35
Titanium .25 21.9 .523 1941
Scandium .18 15.8 1814
Neodymium .156 1297
Mercury .10 8.30 .140 234
Manganese .062 7.81 1519
Germanium .00019 1211
Diamondiso 10 3320
Diamond e-16 2200 .509
Nanotube 10 3500 Carbon nanotube. Electric conductivity = e-16 laterally
Tube bulk 200 Carbon nanotubes in bulk
Graphene 10 5000
Graphite 2 400 .709 Natural graphite
Al Nitride e-11 180
Brass 1.5 120
Steel 45 Carbon steel
Bronze .65 40
Steel Cr .15 20 Stainless steel (usually 10% chromium)
Quartz (C) 12 Crystalline quartz. Thermal conductivity is anisotropic
Quartz (F) e-16 2 Fused quartz
Granite 2.5
Marble 2.2
Ice 2
Concrete 1.5
Limestone 1.3
Soil 1
Glass e-12 .85
Water e-4 .6
Seawater 1 .6
Brick .5
Plastic .5
Wood .2
Wood (dry) .1
Plexiglass e-14 .18
Rubber e-13 .16
Snow .15
Paper .05
Plastic foam .03
Air 5e-15 .025
Nitrogen .025 1.04
Oxygen .025 .92
Silica aerogel .01
Siemens: Amperes^2 Seconds^3 / kg / meters^2 = 1 Ohm^-1
For most metals,
Electric conductivity / Thermal conductivity ~ 140 J/g/K
Teslas
Field generated by brain 10-12
Wire carrying 1 Amp .00002 1 cm from the wire
Earth magnetic field .0000305 at the equator
Neodymium magnet 1.4
Magnetic resonance imaging machine 8
Large Hadron Collider magnets 8.3
Field for frog levitation 16
Strongest electromagnet 32.2 without using superconductors
Strongest electromagnet 45 using superconductors
Neutron star 1010
Magnetar neutron star 1014
The critical electric field for electric breakdown for the following materials is:
MVolt/meter
Air 3
Glass 12
Polystyrene 20
Rubber 20
Distilled water 68
Vacuum 30 Depends on electrode shape
Diamond 2000
Relative permittivity is the factor by which the electric field between charges is decreased relative to vacuum. Relative permittivity is dimensionless. Large permittivity is desirable for capacitors.
Relative permittivity
Vacuum 1 (Exact)
Air 1.00059
Polyethylene 2.5
Sapphire 10
Concrete 4.5
Glass ~ 6
Rubber 7
Diamond ~ 8
Graphite ~12
Silicon 11.7
Water (0 C) 88
Water (20 C) 80
Water (100 C) 55
TiO2 ~ 150
SrTiO3 310
BaSrTiO3 500
Ba TiO3 ~ 5000
CaCuTiO3 250000
A ferromagnetic material amplifies a magnetic field by a factor called the "relative permeability".
Relative Magnetic Maximum Critical
permeability moment frequency temperature
(kHz) (K)
Metglas 2714A 1000000 100 Rapidly-cooled metal
Iron 200000 2.2 1043
Iron + nickel 100000 Mu-metal or permalloy
Cobalt + iron 18000
Nickel 600 .606 627
Cobalt 250 1.72 1388
Carbon steel 100
Neodymium magnet 1.05
Manganese 1.001
Air 1.000
Superconductor 0
Dysprosium 10.2 88
Gadolinium 7.63 292
EuO 6.8 69
Y3Fe5O12 5.0 560
MnBi 3.52 630
MnAs 3.4 318
NiO + Fe 2.4 858
CrO2 2.03 386
Resistivity in 10^-9 Ohm Meters
293 K 300 K 500 K
Beryllium 35.6 37.6 99
Magnesium 43.9 45.1 78.6
Aluminum 26.5 27.33 49.9
Copper 16.78 17.25 30.9
Silver 15.87 16.29 28.7
Gauge Diameter Continuous 10 second 1 second 32 ms Resistance
mm current current current current
Ampere Ampere Ampere Ampere Ohm/meter
0 8.3 125 1900 16000 91000 .00032
2 6.5 95 1300 10200 57000 .00051
4 5.2 70 946 6400 36000 .00082
6 4.1 55 668 4000 23000 .00130
12 2.0 20 235 1000 5600 .0052
18 1.02 10 83 250 1400 .021
24 .51 3.5 29 62 348 .084
30 .255 .86 10 15 86 .339
36 .127 .18 4 10 22 1.361
40 .080 1 1.5 8 3.441
 |
|---|