2(OH)6.jpg)



2(OH)6.jpg) |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
 |
 |
 |
 |
|---|---|---|---|---|
 |
|---|
The earliest metals were gold and silver, the only ones that occur naturally in pure form.
Iron can occasionally be found as iron meteorites.
Copper was discovered around 7000 BCE by smelting copper minerals in a wood fire.
Around 3200 BCE it was found that copper is strenghened by tin, and this is called bronze.
Around 2000 BCE it was found that copper is also strengthed by zinc, and this is called brass.
The earliest metals were smeltable with a wood fire and they consist of copper, lead, silver, tin, zinc, and mercury.
They come from the following minerals:
Gold and silver were known since antiquity, but gold mining didn't start until 6000 BC, and silver smelting didn't
start until 4000 BC.
The minerals that were used by ancient civilizations to smelt metal are:
The next metal to be discovered was iron (c. 1200 BC), which requires a bellows-fed coal fire to smelt.
No new metals were discovered until cobalt in 1735. Once cobalt was discovered, it was realized that
new minerals may have new metals, and the race was on to find new minerals. This yielded
nickel, chromium, manganese, molybdenum, and tungsten.
Chromium is lighter and stronger than steel and it was discovered in 1797. It
satisfies the properties of "Valyrian steel" from Game of Thrones. There's no reason chromium couldn't have
been discovered earlier.
Coal smelting can't produce the metals lighter than chromium. For these you
need electrolysis. The battery was invented in 1799, enabling electrolysis, and
the lighter metals were discovered shortly after. These include aluminum, magnesium, titanium,
and beryllium.
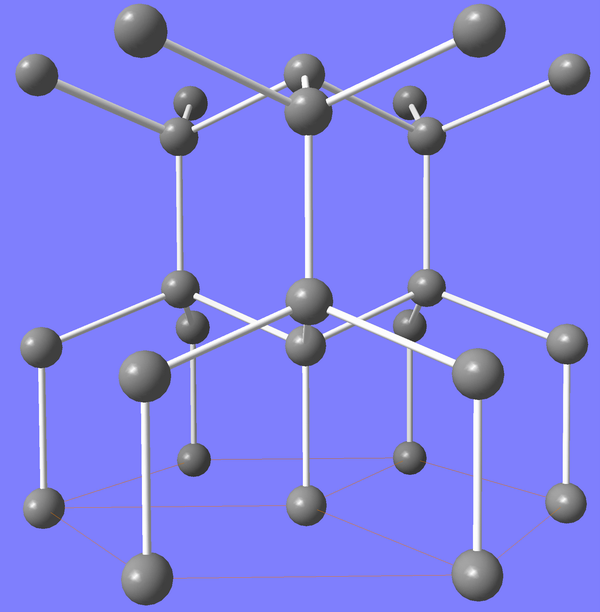
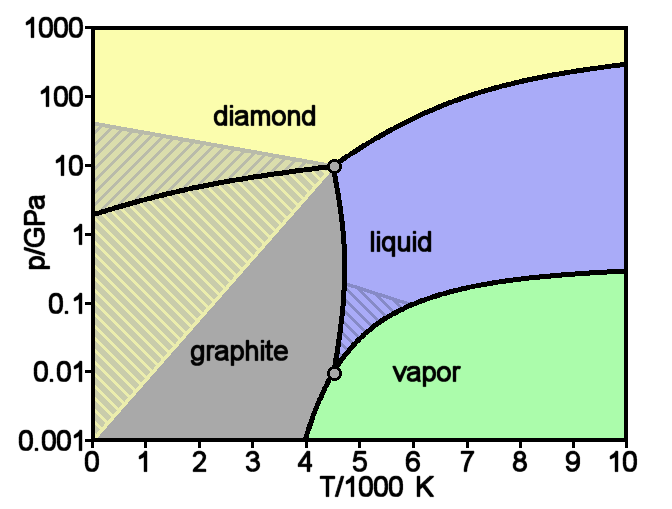
Carbon fiber eclipses metals. The present age could be called the carbon age. The carbon age became
mature in 1987 when Jimmy Connors switched from a wood to a carbon racket.
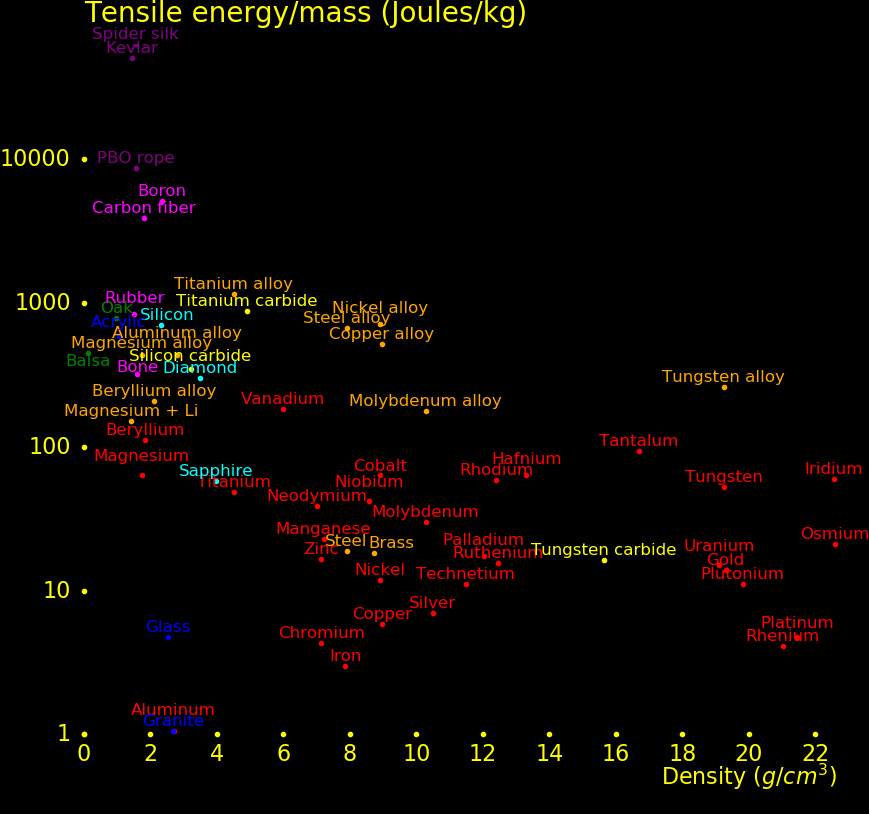
The plot shows the strength of materials.
Wood rivals alloys for strength.
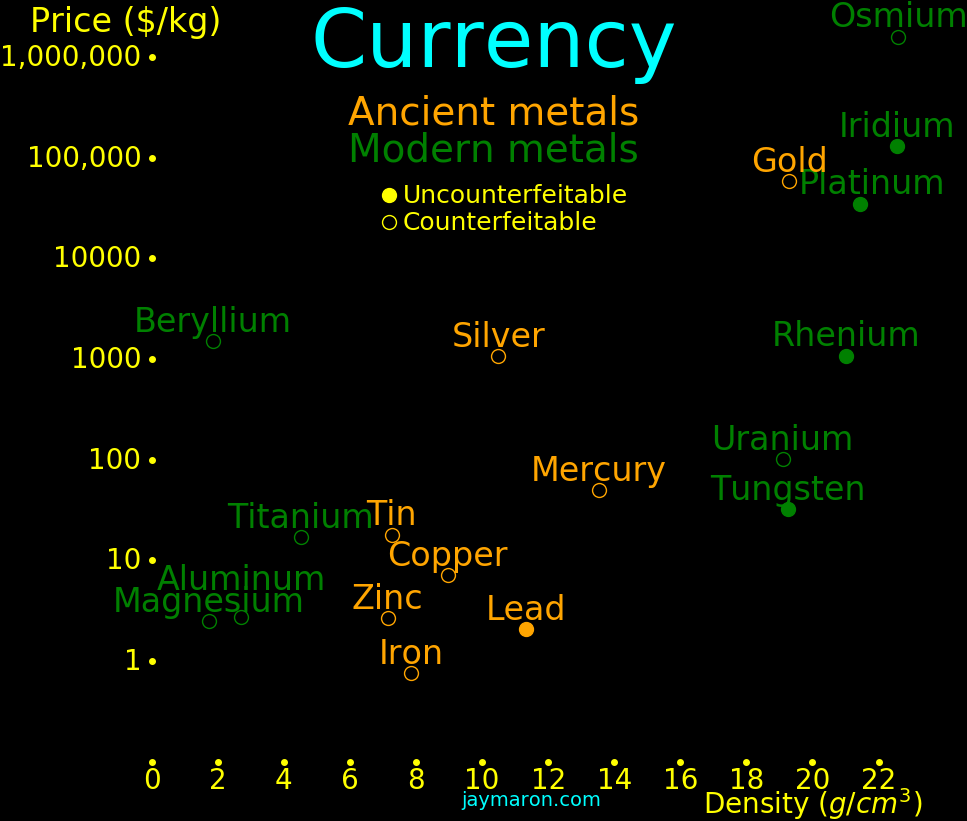
Gold was the densest element known until the discovery of platinun in 1735. It
was useful as an uncounterfeitable currency until the discovery of tungsten in
1783, which has the same density as gold. Today, we could use iridium,
platinum, or rhenium as an uncounterfeitable currency.
Prior to 1800, metals were obtained by smelting minerals, and the known metals
were gold, silver, copper, iron, tin, zinc, mercury, cobalt, manganese,
chromium, molybdenum, and tungsten. Elements to the left of chromium titanium
and scandium cant's be obtained by smelting, and neither can aluminum,
magnesium, and beryllium. They require electrolysis, which was enabled by
Volta's invention of the battery in 1799.
Prior to 1800, few elements were known in pure form. Electrolyis enabled the
isolation of most of the rest of the elements. The periodic table then became
obvious and was discovered by Mendeleev 1871. The battery launched modern
chemistry, and the battery could potentially have been invented much earlier.
Electrolysis enabled the isolation of sodium and potassium in 1807, and these were used
to smelt metals that can't be smelted with carbon.
These elements are not necessarily on the Science Olympiad list.
We list minerals by element, with the most abundant mineral for each element listed first.
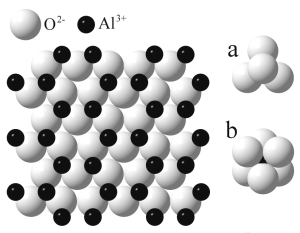
Corundum is a crystalline form of aluminium oxide (Al2O3). It is transparent in
its pure form and can have different colors when metal impurities are
present.
We use relative units for strength and decay time
Hydrogen forms molecules with all elements except the noble gases, osmium,
iridium, promethium, francium, and radium. This makes it a benchmark for
determining the number of bonds that each element forms, as well as the
strength of each element's attraction for electrons.
The "valence number" is the number of bonds that an element can form. The
valence number for each element can be inferred from the table of hydrides.
Elements in the same column of the periodic table have the same valence number.
The following table shows the valence number for each column of the periodic table.
The table gives the most common oxidation number for each metal.
The oxides of copper are:
The oxides of iron are:
In the table, the "e-" column denotes the number of electrons
given by the element to oxygen atoms.
Every atom attracts electrons and the electronegativity table shows the
relative energy released when the atom captures an electron. The elements in
the upper right are the most electron-hungry.
In the reaction H + H + O → H2O,
The oxygen steals an electron from each of the two hydrogens. It is able to do this
because the electrons are at a lower energy with oxygen than with hydrogen.
Most chemical reactions involve elements on the left side of the periodic table
giving electrons to elements on the right side.
The table gives the energy to remove a hydrogen from a hydride molecule.
This is the data used to construct the electronegativity table.
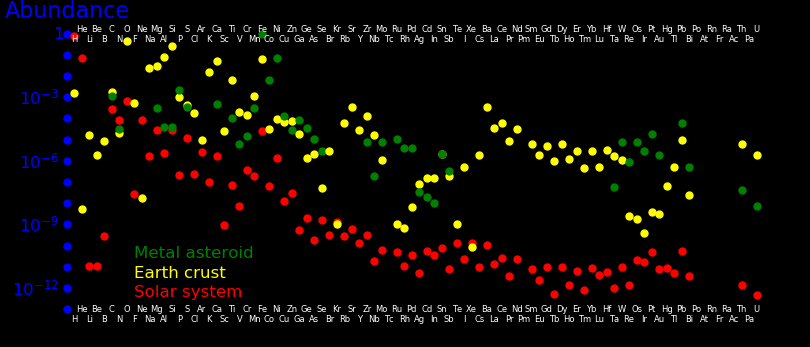
The abundance of elements in the Earth's crust is:
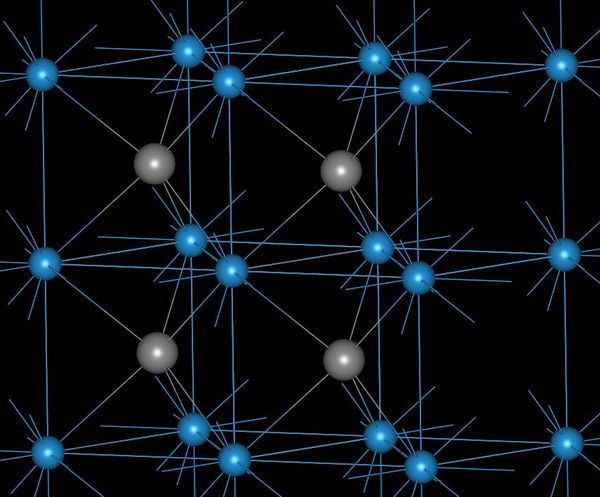
A metal asteroid is a representation of the Earth's core. Platinum group metals
and dense metals tend to sink to the core.
Tungsten is dense but escapes sinking to the core by mineralizing. This
is why tungsten is cheap and all other dense metals are expensive.
The core concentrates iron and other siderophiles. The concentration of iron is larger
in the core than in the crust.
The chart shows the degree to which the core concentrates elements, with iron normalized
to "1".
Platinum is more dense than iron and is hence more likely to sink to the Earth's
core than iron. This is reflected in the "core amplification factor".
For most minerals, the economic importance is the metal it contains.
The metals from manganese to zinc can be extracted by smelting with carbon.
The metals from potassium to vanadium can't be extracted by smelting and instead require
electrolysis.
Many elements are obtained as byproducts from other elements. For example, gallium is a
byproduct of aluminum extraction.
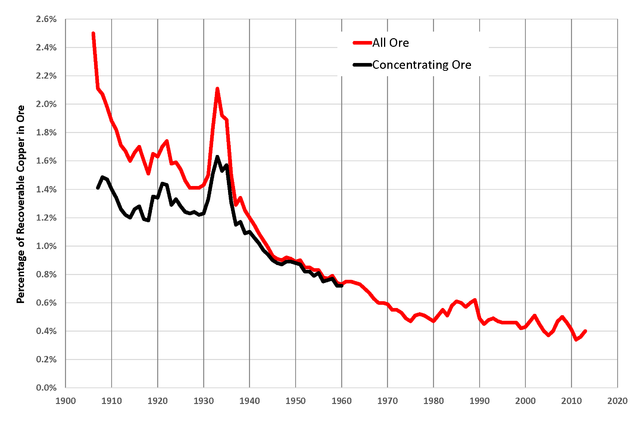
Element price tends to be inversely proportional to the concentration of the
element in ore. Miners search for good ore, ore that has a high concentration
of the target element.
![]()













.jpg)










Alloys can be much stronger than pure metals.

Discovery Method of Source
(year) discovery
Carbon Ancient Naturally occuring
Gold Ancient Naturally occuring
Silver Ancient Naturally occuring
Sulfur Ancient Naturally occuring
Lead -6500 Smelt with carbon Galena PbS
Copper -5000 Smelt with carbon Chalcocite Cu2S
Bronze (As) -4200 Copper + Arsenic Realgar As4S4
Tin -3200 Smelt with carbon Calamine ZnCO3
Bronze (Sn) -3200 Copper + Tin
Brass -2000 Copper + Zinc Sphalerite ZnS
Mercury -2000 Heat the sulfide Cinnabar HgS
Iron -1200 Smelt with carbon Hematite Fe2O3
Arsenic 1250 Heat the sulfide Orpiment As2S3
Zinc 1300 Smelt with wool Calamine ZnCO3 (smithsonite) & Zn4Si2O7(OH)2·H2O (hemimorphite)
Antimony 1540 Smelt with iron Stibnite Sb2S3
Phosphorus 1669 Heat NaPO3 Excrement
Cobalt 1735 Smelt with carbon Cobaltite CoAsS
Platinum 1735 Naturally occuring
Nickel 1751 Smelt with carbon Nickeline NiAs
Bismuth 1753 Isolated from lead
Hydrogen 1766 Hot iron + steam Water
Oxygen 1771 Heat HgO
Nitrogen 1772 Isolated from air
Manganese 1774 Smelt with carbon Pyrolusite MnO2
Molybdenum 1781 Smelt with carbon Molybdenite MoS2
Tungsten 1783 Smelt with carbon Wolframite (Fe,Mn)WO4
Chromium 1797 Smelt with carbon Crocoite PbCrO4
Palladium 1802 Isolated from Pt
Osmium 1803 Isolated from Pt
Iridium 1803 Isolated from Pt
Rhodium 1804 Isolated from Pt
Sodium 1807 Electrolysis
Potassium 1807 Electrolysis
Magnesium 1808 Electrolysis Magnesia MgCO3
Cadmium 1817 Isolated from zinc
Lithium 1821 Electrolysis of LiO2 Petalite LiAlSi4O10
Zirconium 1824 Smelt with potassium Zircon ZrSiO4
Aluminum 1827 Smelt with potassium
Silicon 1823 Smelt with potassium
Beryllium 1828 Smelt with potassium Beryl Be3Al2Si6O18
Thorium 1929 Smelt with potassium Gadolinite (Ce,La,Nd,Y)2FeBe2Si2O10
Vanadium 1831 Smelt VCl2 with H2 Vanadinite Pb5(VO4)3Cl
Uranium 1841 Smelt with potassium Uranite UO2
Ruthenium 1844 Isolated from Pt
Tantalum 1864 Smelt with hydrogen Tantalite [(Fe,Mn)Ta2O6]
Niobium 1864 Smelt with hydrogen Tantalite [(Fe,Mn)Ta2O6]
Fluorine 1886 Electrolysis
Helium 1895 From uranium ore
Titanium 1910 Smelt with sodium Ilmenite FeTiO3
Hafnium 1924 Isolated from zirconium
Rhenium 1928 Isolated from Pt
Scandium 1937 Electrolysis Gadolinite FeTiO3
-384 -322 Aristotle. Wrote "Meteorology"
-370 -285 Theophrastus. Wrote "De Mineralibus"
77 Pliny the Elder publishes "Natural History"
973 1050 Al Biruni. Published "Gems"
1546 Georgius Agricola publishes "On the Nature of Rocks"
1556 Georgius Agricola publishes "On Metals"
1609 de Boodt publishes a catalog of minerals
1669 Brand: Discovery of phosphorus
1714 John Woodward publishes "Naturalis historia telluris illustrata & aucta", a mineral catalog
1735 Brandt: Discovery of cobalt
1777 Lavoisier: Discovery of sulfur
1778 Lavoisier: Discovery of oxygen and prediction of silicon
1783 Lavoisier: Discovery of hydrogen
1784 T. Olof Bergman publishes "Manuel du mineralogiste, ou sciagraphie du regne mineral",
and founds analytical chemistry
1778 Lavoisier: Discovery of oxygen
1801 Rene Just Huay publishes "Traite de Mineralogie", founding crystallography
1811 Avogadro publishes "Avogadro's law"
1860 The Karlsruhe Congress publishes a table of atomic weights
1869 Mendeleev publishes the periodic table
.jpg)













2(OH)6.jpg)













_.jpg)







.jpg)






.jpg)






_3.jpg)












-178918.jpg)






























Color Colorant carat ($)
Painite 55000 CaZrAl9O15(BO3)
Diamond Clear 1400 C
Ruby Red Chromium 15000 Al2O3
Sapphire Blue Iron 650 Al2O3
Sapphire yellow Titanium Al2O3
Sapphire Orange Copper Al2O3
Sapphire Green Magnesium Al2O3
Emerald Green Chromium Be3Al2(SiO3)6
Beryl Aqua Iron Be3Al2(SiO3)6 AKA "aquamarine"
Morganite Orange Manganese 300 Be3Al2(SiO3)6
Topaz Topaz Al2SiO4(F,OH)2
Spinel Red Red MgAl2O4
Quartz Clear SiO2
Amethyst Purple Iron SiO2
Citrine Yellow SiO2
Zircon Red ZrSiO4
Garnet Orange [Mg,Fe,Mn]3Al2(SiO4)3 & Ca3[Cr,Al,Fe]2(SiO4)3
Garnet Blue 1500 [Mg,Fe,Mn]3Al2(SiO4)3 & Ca3[Cr,Al,Fe]2(SiO4)3
Opal SiO2·nH2O
Opal Black 11000 SiO2·nH2O
Jet Black Lignite
Peridot Green (Mg,Fe)2SiO4
Pearl White CaCO3
Jade Green NaAlSi2O6
Amber Orange Resin
Strength Decay time
SrOAl2O3 Strontium aluminate Light green 1 1
ZnS Zinc sulfide Dark green .1 .1
CaS Calcium sulfide Red
SrS Strontium sulfide
1 2 3 4 3 2 1 0
Hydrogen Helium
Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon
Sodium Magnesium Aluminum Silicon Phosphorus Sulfur Chlorine Argon
Potassium Calcium Gallium Germanium Arsenic Selenium Bromine Krypton
Lithium 1 Potassium 1
Beryllium 2 Calcium 2
Scandium 3
Sodium 1 Titanium 4
Magnesium 2 Vanadium 4
Aluminum 3 Chromium 4
Manganese 4
Iron 3
Cobalt 2
Nickel 2
Copper 3
Zinc 2



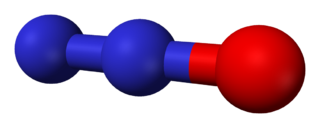
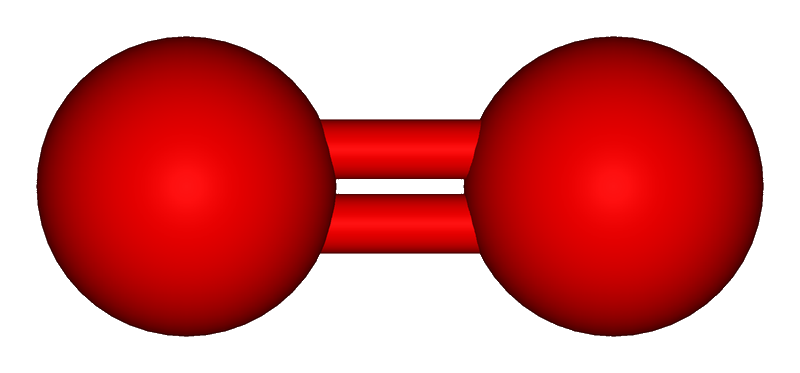
1 electron: Cu2O Copper(I) Oxide. Cuprous oxide. Red paint
2 electrons: CuO Copper(II) Oxide. Cupric oxide. Black color
3 electrons: Cu2O3 Copper(III) Oxide
"Electrons" refers to the number of electrons that each copper atom gives to the
oxygen atoms.
3 electons: Fe2O3 Iron(III) Oxide. Ferric oxide. Most common form
2 electrons: FeO Iron(II) oxide. Rare
2 or 3 electrons: Fe3O4 Iron(II,III) Oxide. Magnetite
e-
1 H 1 H2O Water
2 He 0 Does not react with oxygen
3 Li 1 Li2O
4 Be 2 BeO Beryllium oxide
5 B 3 B2O3 Boron trioxide. Most common form. High conductivity
1/3 B6O Boron suboxide. High conductivity and hardness
6 C 4 CO2 Carbon dioxide
2 CO Carbon monoxide. Toxic. Displaces oxygen from hemoglobin
7 N 1 N2O Nitrous oxide. Laughing gas
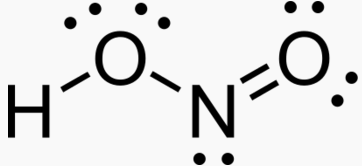
2 NO Nitric oxide. Gas. Signaling molecule. Decomposes in air to NO2
4 NO2 Nitrogen dioxide. Toxic gas
4 N2O4 Dinitrogen tetroxide
5 N2O5 Dinitrogen pentoxide
8 O O2 Oxygen
O3 Ozone
9 F -2 OF2 Oxygen difluoride
10 Ne 0 Does not react with oxygen
11 Na 1 Na2O Sodium oxide
12 Mg 2 MgO Magnesium oxide
13 Al 3 Al2O3 Aluminum(III) oxide. Aluminum oxide. Most common form
2 AlO Aluminum(II) oxide. Aluminum monoxide
1 Al2O Aluminum(I) oxide
14 Si 4 SiO2 Quartz
15 P 4 P2O4 Diphosphorus tetroxide
3 P4O6 Phosphorus trioxide. Phosphorus(III) oxide. Stable. Reacts with water
5 P4O10 Phosphorus pentoxide
16 S 6 SO3 Sulfur trioxide. Component of acid rain
4 SO2 Sulfur dioxide. Toxic gas
2 SO Sulfur monoxide. Unstable
17 Cl 4 ClO2 chlorine dioxide
2 ClO Foe of the ozone layer
1 Cl2O Dichlorine monoxide. Unstable. Explosive
18 Ar 0 Does not react with oxygen
19 K 1 K2O Potassium oxide
20 Ca 2 CaO Calcium oxide Quicklime
21 Sc 3 Sc2O3 Scandium(III) oxide. Ceramic
22 Ti 4 TiO2 Titanium dioxide. Most common form
3 Ti2O3 Dititanium trioxide
2 TiO Titanium monoxide. Corundum structure. Tistarite ore (extremely rare)
23 V 5 V2O5 Vanadium(V) oxide. Rare mineral
4 VO2 Vanadium(IV) oxide
3 V2O3 Vanadium(III) oxide. Morphs in air to V2O4
2 VO Vanadium(II) oxide
24 Cr 2 CrO Chromium(II) oxide
3 Cr2O3 Chromium(III) oxide. Eskolaite ore
4 CrO2 Chromium dioxide
6 CrO3 Chromium trioxide
25 Mn 7 Mn2O7 Manganese(VII) oxide. Extremely unstable
6 MnO3 Manganese(VI) oxide
4 MnO2 Manganese dioxide. Most common form
3 Mn2O3 Manganese(III) oxide
8/3 Mn3O4 Manganese(II,III) oxide
2 MnO Manganese(II) oxide. Rare mineral
26 Fe 3 Fe2O3 Iron(III) Oxide. Ferric oxide. Most common form
2 FeO Iron(II) oxide. Rare
8/3 Fe3O4 Iron(II,III) Oxide. Magnetite
27 Co 3 Co2O3 Cobalt(II) oxide. Cobaltic oxide
2 CoO Cobalt(II) oxide. Cobaltous oxide
8/3 Co3O4 Cobalt(II,IIIs) oxide. Cobaltous oxide
28 Ni 2 NiO Nickel(II) oxide
29 Cu 1 Cu2O Copper(I) Oxide. Cuprous oxide. Red paint
2 CuO Copper(II) Oxide. Cupric oxide. Black color. Common ore
3 Cu2O3 Copper(III) Oxide
30 Zn 2 ZnO Zinc oxide
30 Ga 1 GaO2 Gallium(I) oxide
Ga 3 Ga2O3 Gallium(III) oxide
32 Ge 2 GeO2 Germanum oxide
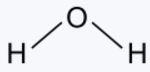
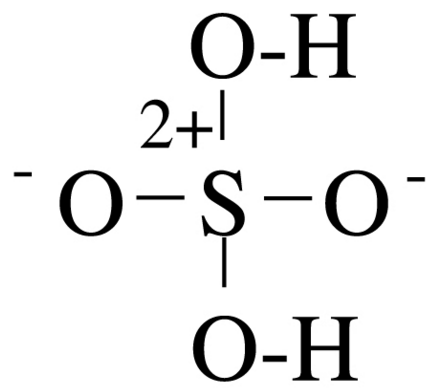
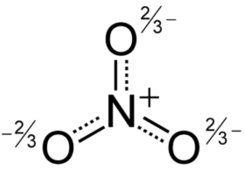
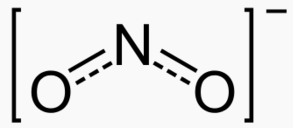
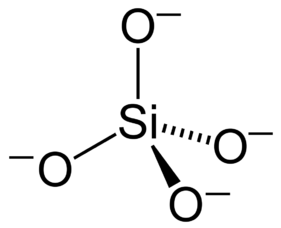
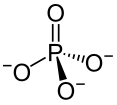
Type Example
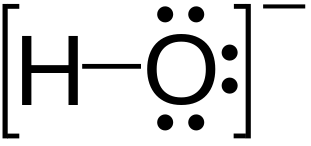
Hydroxide HOH Hydrogen hydroxide
Hypofluorite HFO Hypoflourous acid
Hypochlorite HClO Hypochlorous acid
Peroxide H2O2 Hydrogen peroxide
Carbide WC Tungsten carbide
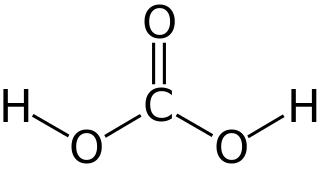
Oxide H2O Hydrogen oxide
Fluoride HF Hydrogen fluoride
Silicide H4Si Hydrogen silicide
Phosphide H3P Hydrogen phosphide
Sulfide H2S Hydrogen sulfide
Chloride HCl Hydrogen chloride
Arsenide H3As Hydrogen arsenide
Selenide H2Se Hydrogen selenide
Bromide HBr Hydrogen bromide
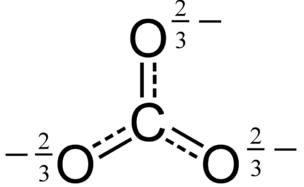
Carbonate H2CO3 Carbonic acid
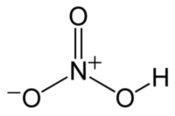
Nitrate HNO3 Nitric acid
Aluminate H5AlO4 Hydrogen aluminate
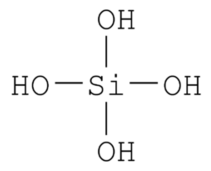
Silicate H4SiO4 Silicic acid
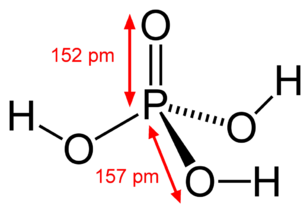
Phosphate H3PO4 Phosphoric acid
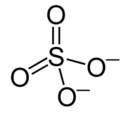
Sulfate H2SO4 Sulfuric acid
Chlorate HClO3 Hydrogen chlorate
Perchlorate HClO4 Hydrogen perchlorate
Germanate H4GeO4 Hydrogen germanate
Arsenate H3AsO4 Arsenic acid
Selenate H2SeO4 Hydrogen selenate
Bromate HBrO3 Hydrogen bromate
Tellurate H2TeO4 Hydrogen tellurate
Iodate HIO3 Hydrogen iodate
Nitrite HNO2 Nitrous acid
Chlorite HClO2 Hydrogen chlorite

![]()
Carbon atoms per metal atom
Boron 1/4
Silicon 1
Titanium 1
Beryllium 1/2
Zirconium 1/2
Tantalum 1
Tungsten 1
Aluminum 3/4

Element Molecule Bond energy
(eV)
H H2 4.52
Li LiH 2.56
Be BeH2 2.35
B BH3 3.43
C CH4 3.52
N NH3 3.26
O H2O 4.41
F HF 4.90
Na NaH 2.09
Mg MgH2 2.04
Al AlH3 2.96
Si SiH4 3.10
P PH3 3.56
S H2S 3.57
Cl HCl 4.48
K KH 1.90
Ca CaH2 1.74
Ga ?
Ge 3.36
As 2.82
Se SeH2 3.17
Br HBr 3.80
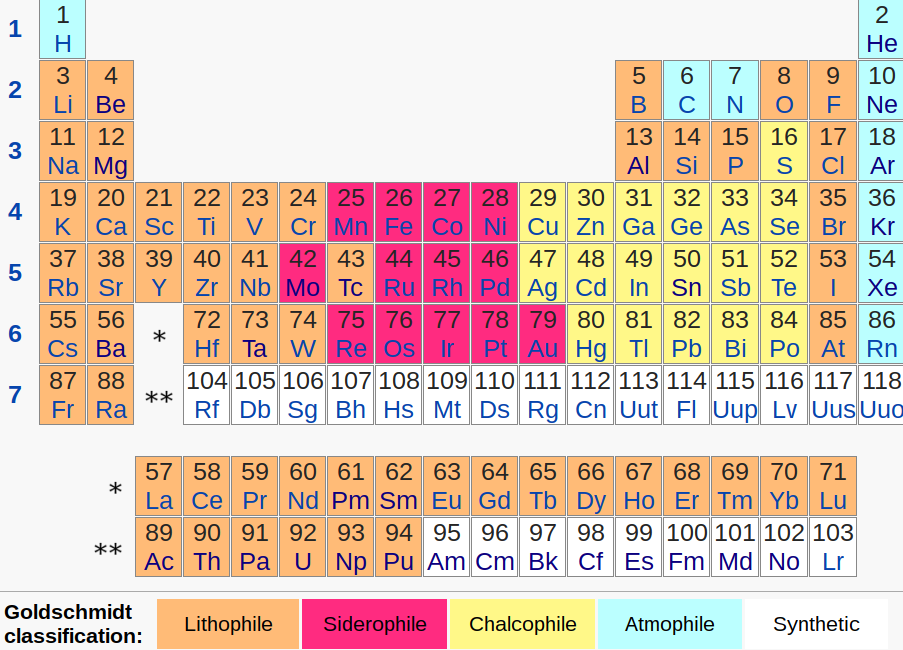
Siderophile: Iron-living. Tends to sink to the core along with iron.
Lithophile: Rock-loving. Tends to become included in rock and escapes sinking to the core.
Chalcophile: Ore-loving. Tends to combine with oxygen and sulfur and escapes sinking to the core.
Atmophile: Is a gas at room temperature and tends to escape the crust into the atmosphere.
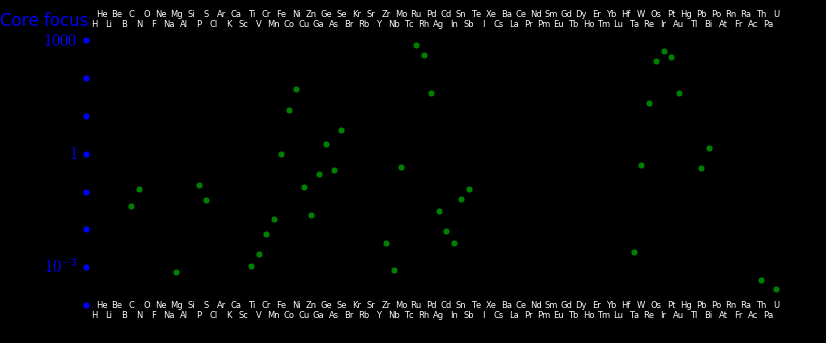
Platinum abundance in the crust = cPt = .004 ppm
Iron abundance in the crust = cFe = 63000 ppm
Platinum abundance in the core = CPt = 19 ppm
Iron abundance in the core = CFe =910000 ppm
Crust platinum/iron = cPt/cFe = .000000063
Core platinum/iron = CPt/CFe = .000021
Core amplification factor = APt = (CPt/CFe) / (cPtcFe) = 329
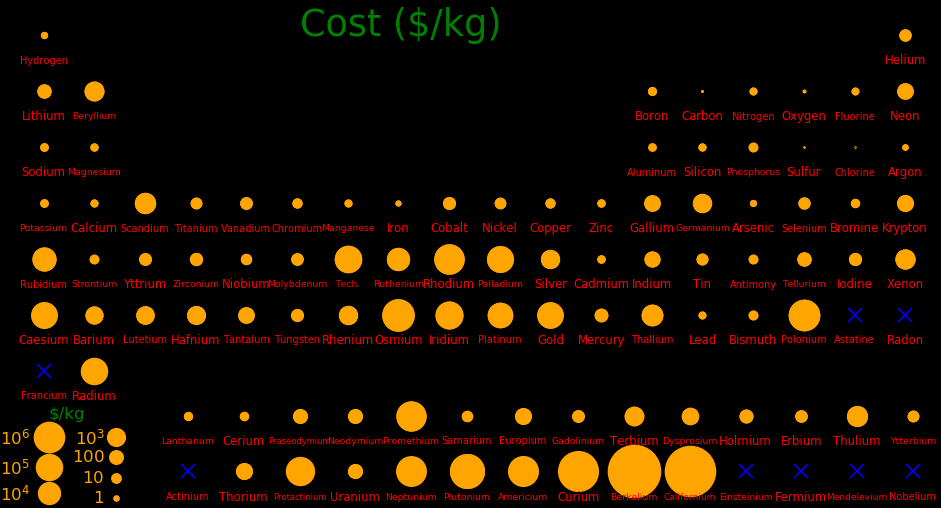
Chief ore Chief uses
Lithium Spodumene Lithium-ion batteries LiAl(SiO3)2
Lepidolite K(Li,Al)3(Si,Al)4O10(F,OH)2
Beryllium Beryl Lightweight metal, copper alloy Be3Al2(SiO3)6
Boron Ulexite Glass, ceramics, fiberglass NaCa[B5O6(OH)6]·5H2O
Colemanite Ca2B6O11·5H2O
Kernite Na2B4O6(OH)2·3H2O
Tincal Na2B4O7·10H2O
Fluorine Fluorite UF6, organofluorides, circuit breakers CaF2
Magnesium Periclase Al alloy, die-asting, Fe smelting MgO
Magnesite MgCO3
Dolomite CaMg(CO3)2
Aluminum Bauxite Structural metal Al(OH)3, AlO(OH)
Phosphorus Phosphate rock Fertilizer
Potassium Brine Fertilizer
Scandium Thortvetite Aluminum alloy (Sc,Y)2Si2O7
Titanium Rutile Pigments, alloys, aircraft TiO2
Vanadium Vanadinite Steel alloy Pb5(VO4)3Cl
Chromium Chromite Steel alloy FeCr2O4
Manganese Pyrolusite Steel alloy, Aluminum alloy MnO2
Braunite Mn+2Mn+36)SiO12
Psilomelane (Ba,H2O)2Mn5O10
Rhodochrosite MnCO3
Iron Hematite Structural metal Fe2O3
Cobalt Cobaltite Li-ion batteries, steel alloy CoAsS
Nickel Millerite Steel alloy NiS
Nickeline NiAs
Copper Chalcocite Conductors Cu2S
Zinc Sphalerite Brass ZnS
Smithsonite ZnCO3
Hemimorphite Zn4(Si2O7)(OH)2·H2O
Gallium Aluminum ore Semiconductors Al(OH)3 and AlO(OH)
Germanium Germanite Fiber optics Cu26Fe4Ge4S32
Arsenic Realgar Pesticides As4S4
Arsenopyrite FeAsS
Selenium Chalcocite Glass, Bi & Brass alloy Cu2S
Bromine Brine Flame retardant, organobromides
Rubidium Lepidolite Fireworks K(Li,Al)3(Si,Al)4O10(F,OH)2
Strontium Celestite Cathode ray tubes, fireworks SrSO4
Yttrium Rare-earth ore CRTs
Zirconium Zircon Nuclear reactors ZrSiO4
Niobium Niobite Steel alloy, superalloy, superconductors
Molybdenum Molybdenite Steel alloy MoS2
Ruthenium Nickel ore Electrical contacts, catalysts
Rhodium Nickel ore Catalyst
Palladium Nickel ore Catalyst
Silver Acanthite Currency, conductors Ag2S
Argentite
Cadmium Zinc ore Batteries, pigments, electroplating
Greenockite CdS
Indium Cu & Zn ore LCDs, semiconductors, solder Sphalerite and chalcopyrite
Tin Cassiterite Bronze and solder SnO2
Antimony Stibnite Flame retardant, Pb alloy Sb2S3
Tellurium Chalcocite Fe, Cu, Pb alloy Cu2S
Iodine Brine Organoiodides Ca(IO3)2
Lautarite Ca(IO3)2
Dietzeite 7Ca(IO3)2·8CaCrO4
Caesium Pollucite Drill fluid (Cs,Na)2Al2Si4O12·2H2O
Barium Barite Drill fluid, CRTs, pigments BaSO4
Rare-earths Mozanite Electronics, solar cells, magnets (Nd,La,Ce)PO4
Hafnium Titanium ore Fission control rods, High-T materials ZrSiO4
Tantalum Tantalite Steel alloy, capacitors, high-T materials (Fe,Mn)Ta2O6
Tungsten Wolframite Carbides, alloys FeWO4
Scheelite CaWO4
Rhenium Moly ore Aircraft turbines
Osmium Platinum ore Pens, electrical contacts
Iridium Platinum ore Crucibles, spark plugs
Platinum Sperrylite Catalyst PtAs2
Gold Native metal Currency, jewelry, electronics
Mercury Cinnabar Chem industry, electronics HgS
Thallium Chalcocite Infrared detectors Cu2S
Lorandite TlAsS2
Crookesite Cu7(Tl,Ag)Se4
Lead Galena Batteries PbS
Anglesite PbSO4
Cerussite PbCO3
Bismuth Bismite Lead replacement, alloys Bi2O3
Bismuthinate Bi2S3
Thorium Rare-earth ore Lightbulb filaments, nuclear power
Uranium Uranite Nuclear power UO2
 |
|---|
Rare elements tend to be valuable.
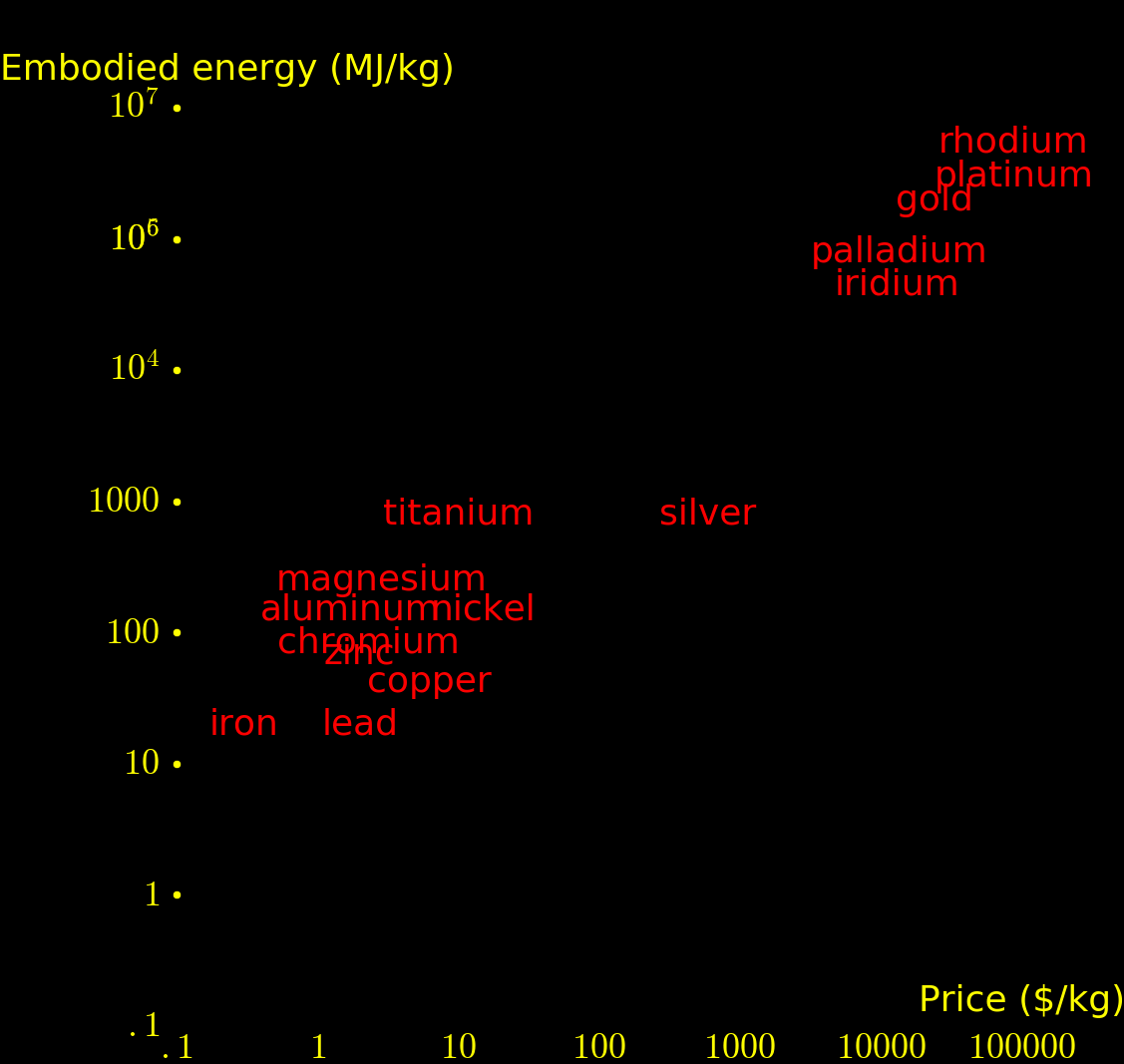
"Embodied energy" is the energy/kg required to extract an element. It's proportional to price and inversely proportional to ore concentration.
 |
|---|
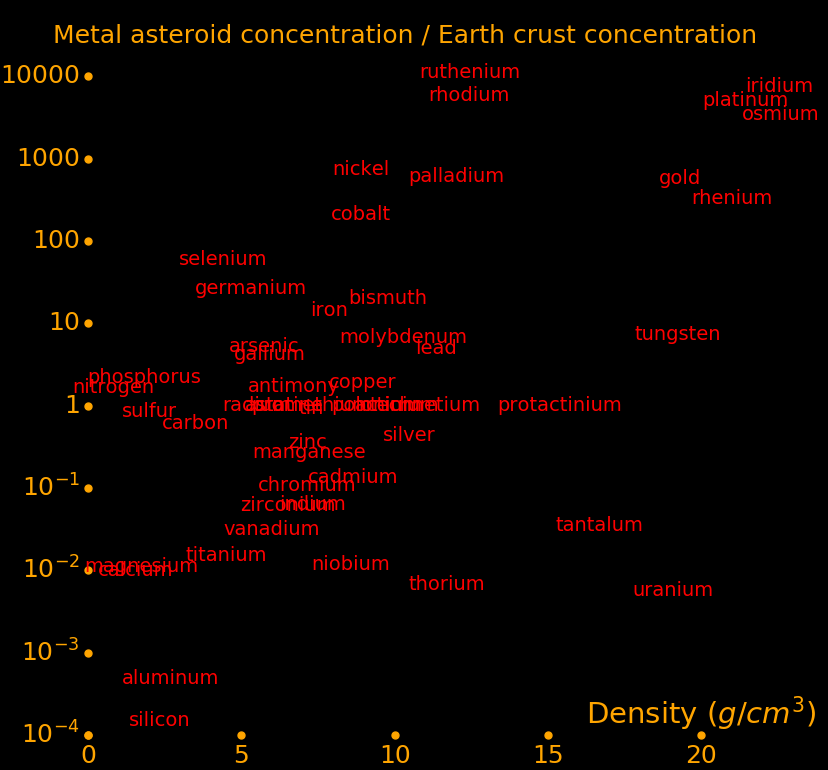
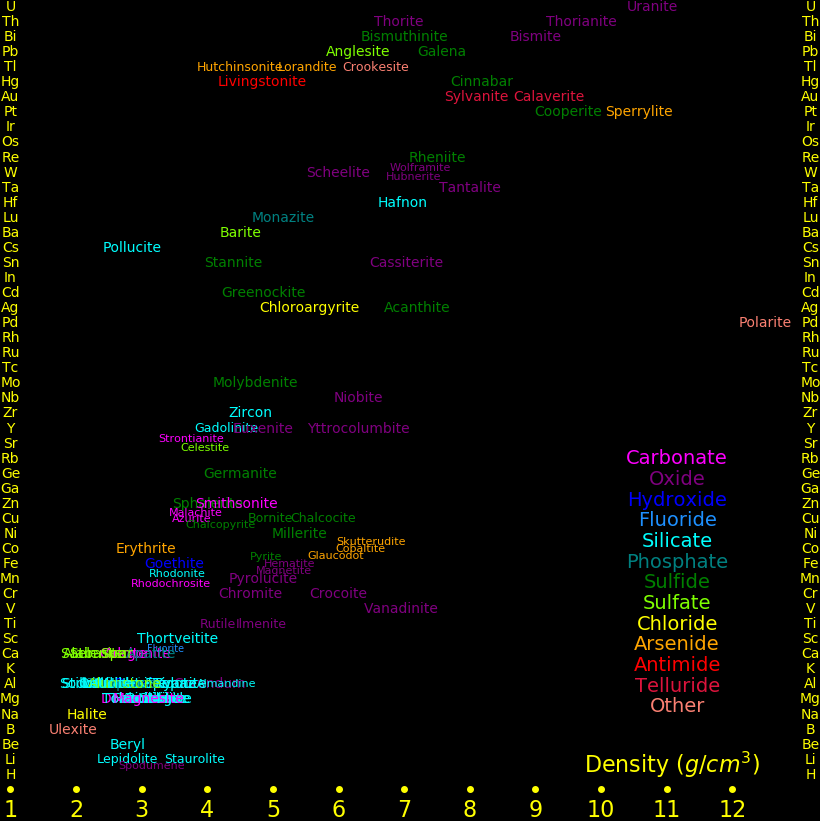
The more dense the metal, the more valuable it tends to be, hence dense minerals tend to be valuable. Prospectors look for dense minerals.
The densest metals are often mineralized with arsenic, antimony, or tellurium, which further increases the density of the mineral.
All minerals with a density larger than 4 grams/cm3 have valuable metals.
Almost all metals beyond calcium in the periodic table are valuable.
In the world of mineralogy, a Holy Grail is to discover a new mineral, because it gets named after you. If you want to discover a new mineral, you must know all known minerals. The same principle applies to bird ID, tree ID, flower ID, insect ID, etc.
 |
|---|
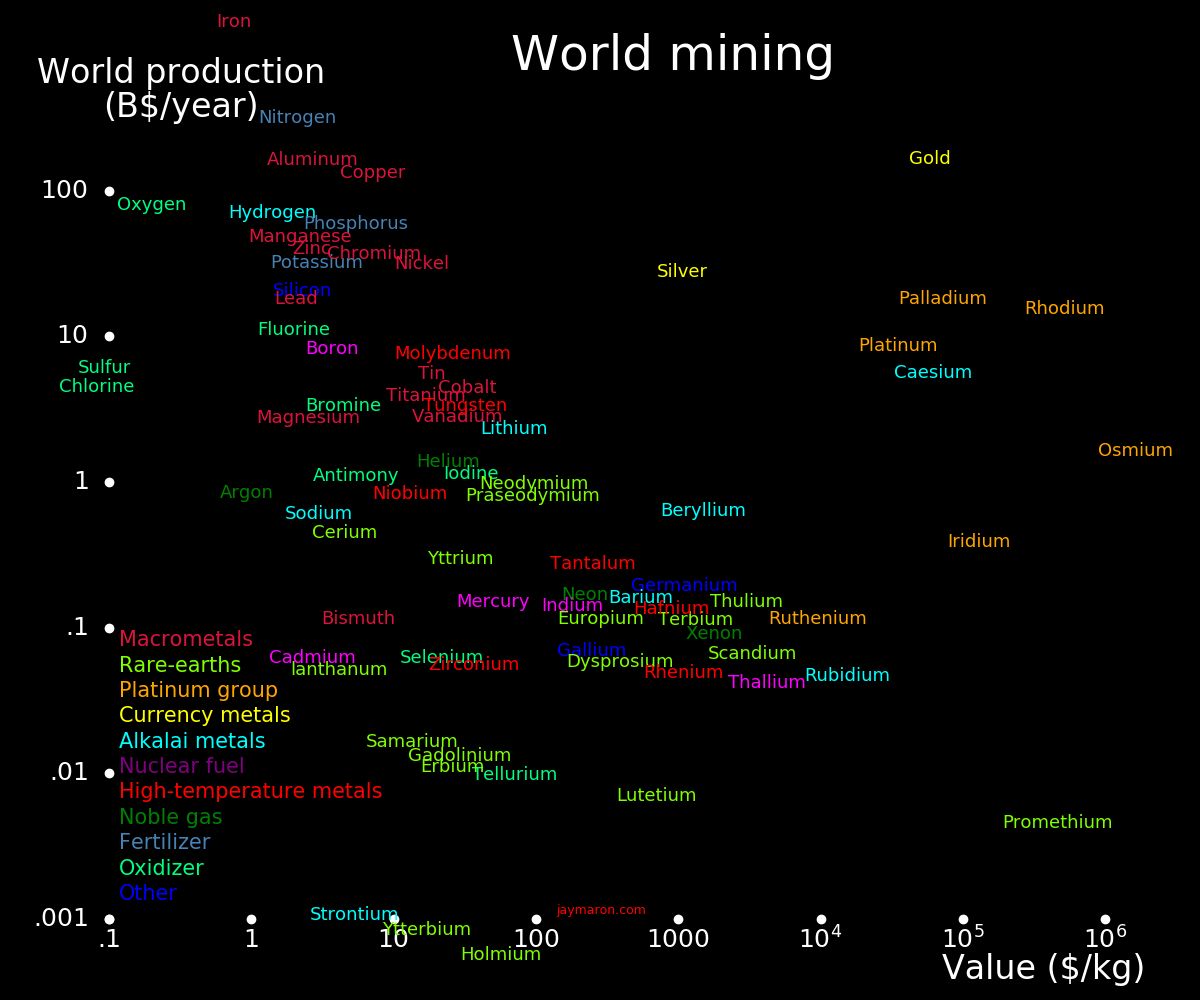
The abundance of an element in the Earth's crust is reflected in the amount the element is mined per year.
 |
|---|
For an element to be useful as currency, it should be toward the right of the plot, and toward the bottom, and it should have industrial importance.
The best mines for platinum-group metals are metal asteroid craters. Metal asteroids are 90% iron, 10% nickel, and rich in platinum-group elements. These mines are rich in nickel ore, and platinum-group minerals tend to accompany nickel minerals. Many platinum-group metals are a byproduct of nickel extraction.
The best metal asteroid mines are Sudbury Canada, and Vredefort South Africa.
Platinum-group metals tend to mineralize with sulfur, tellurium, arsenic, and antimony. Metal asteroid mines are rich sources of these mineral classes.
Minerals can be isolated from rock with dense liquids. Rock has a density between 2.7 and 3.3 gram/cm3, hence anything more dense than this is a mineral. Almost all minerals with a density larger than 3.3 grams/cm3 have valuable elements. In ancient times, gold was mined with mercury. The dense liquids are:
Density Melt Boil Price/Mass
gram/cm3 Celsius Celsius $/kg
Mercury 13.53 -39 357 50
Lead 11.34 327 1749 2.1
BiPbSnCdInTl 10 42 37 Bi=40.3 Pb=22.2 Sn10.7 In17.7 Cd8.1 Tl=1.1
Bismuth 9.78 272 1564 6
Field's metal 8.1 62 97 Bi=32.5%, Sn=16.5%, In=51%
Indium 7.31 157 2072 180
Gallinstan 6.44 -19 208 68.5% Ga, 21.5% In, and 10.0% Sn. Non-toxic
Gallium 5.91 30 2400 245
Caesium formate 2.3 1749 50000 Cs(HCOO) Drilling lubricant
Price ($/kg)
Lead 2.1
Cadmium 2.7
Bismuth 6
Tin 18
Mercury 50
Tellurium 71
Indium 180
Gallium 245
Caesium 62000
Most metals are in oxidized form. The only metals that can be found in
pure form are gold, silver, copper, platinum, palladium, osmium, and iridium.
Smelting is a process for removing the oxygen to produce pure metal.
The ore is heated in a coal furnace and the carbon seizes the oxygen from
the metal. For copper,
For iron, the oxidation state is reduced in 3 stages until the
pure iron is left behind.
The following table gives the temperature required to smelt each element with
carbon.
The farther to the right on the periodic table, the lower the smelting
temperature, a consequence of "electronegativity".
The battery was invented in 1800, launching the field of electrochemistry
and enabling the the isolation of non-carbon-smeltable elements.
Davy used electrolysis in 1807 to isolate sodium and potassium and then he used
these metals to smelt other metals. To smelt beryllium with potassium,
BeO + 2 K ↔ Be + K2O.
Titanium can't be carbon smelted because it forms the carbide Ti3C.
For an expanded discussion of smelting physics, see jaymaron.com/metallurgy.html.
Thermite is smelting with aluminum. For example, to smelt iron with aluminum,
The following table shows reactions that change the oxidation state of a metal.
"M" stands for an arbitrary metal and the magnitudes are scaled to one mole of
O2. The last two columns give the oxidation state of the metal on
the left and right side of the reaction. An oxidation state of "0" is the pure
metal and "M2O" has an oxidation state of "1".
Let MO be a metal oxide for which the Gibbs energy of
CO is larger than MO and the oxygen binds to the metal preferentially over carbon.
The entropies of most metal oxides are similar and so changing the temperature
has little effect on their relative Gibbs energies. CO is special because it
is a gas and hence has a larger entropy than the solid metal oxides. As
temperature increases the Gibbs energy of CO decreases faster than that of MO
and at the critical smelting temperature they are equal. Above this
temperature the oxygen unbinds to the metal and binds to carbon.
For the smelting of cobalt,



Cu2O + C → 2 Cu + CO
At low temperature copper stays in the form of Cu2O and at high
temperature it gives the oxygen to carbon and becomes pure copper.
3 Fe2O3 + C → 2 Fe3O4 + CO
Fe3O4 + C → 3 FeO + CO
FeO + C → Fe + CO
Oxidation state = Number of electrons each iron atom gives to oxygen
Oxidation state
CuO 2
Cu2O 1
Cu 0
Fe2O3 3
Fe3O4 8/3
FeO 2
Fe 0
Smelt Method Year Abundance
(C) (ppm)
Gold <0 * Ancient .0031
Silver <0 * Ancient .08
Platinum <0 * 1735 .0037
Mercury <0 heat -2000 .067
Palladium <0 chem 1802 .0063
Copper 80 C -5000 68
Sulfur 200 * Ancient 420
Lead 350 C -6500 10
Nickel 500 C 1751 90
Cadmium 500 C 1817 .15
Cobalt 525 ? 1735 30
Tin 725 C -3200 2.2
Iron 750 C -1000 63000
Phosphorus 750 heat 1669 10000
Tungsten 850 C 1783 1100
Potassium 850 e- 1807 15000
Zinc 975 C 1746 79
Sodium 1000 e- 1807 23000
Chromium 1250 C 1797 140
Niobium 1300 H 1864 17
Manganese 1450 C 1774 1120
Vanadium 1550 ? 1831 190
Silicon 1575 K 1823 270000
Titanium 1650 Na 1910 66000
Magnesium 1875 e- 1808 29000
Lithium 1900 e- 1821 17
Aluminum 2000 K 1827 82000
Uranium 2000 K 1841 1.8
Beryllium 2350 K 1828 1.9
Smelt: Temperature required to smelt with carbon
Method: Method used to purify the metal when it was first discovered
*: The element occurs in its pure form naturally
C: Smelt with carbon
K: Smelt with potassium
Na: Smelt with sodium
H: Smelt with hydrogen
e-: Electrolysis
heat: Heat causes the oxide to decompose into pure metal. No carbon required.
chem: Chemical separation
Discovery: Year the element was first obtained in pure form
Abundance: Abundance in the Earth's crust in parts per million
Elements with a low carbon smelting temperature were discovered in ancient
times unless the element was rare. Cobalt was discovered in 1735, the first new
metal since antiquity, and this inspired scientists to smelt every known
mineral in the hope that it would yield a new metal. By 1800 all the rare
elements that were carbon smeltable were discovered.

Fe2O3 + 2 Al → 2 Fe + Al2O3
Oxidation state Oxidation state
at left at right
2 M2O ↔ 4 M + O2 1 0
4 MO ↔ 2 M2O + O2 2 1
2 M3O4 ↔ 6 MO + O2 8/3 2
6 M2O3 ↔ 4 M3O4 + O2 3 8/3
2 M2O3 ↔ 4 MO + O2 3 2
2 MO ↔ 2 M + O2 2 0
2/3 M2O3 ↔ 4/3 M + O2 3 0
1 MO2 ↔ 1 M + O2 4 0
2 MO2 ↔ 2 MO + O2 4 2
Standard temperature = T0 = 298 Kelvin = 25 Celsius
Smelting temperature = Tsmelt
Temperature change = t = Tsmelt - T0
Gibbs energy at standard temperature = G
Entropy at standard temperature = S
Gibbs energy at temperature Tsmelt = g = G - t S
CoO Gibbs energy per mole O2 = GCoO = -428.4 kJoule/mole
CO Gibbs energy per mole O2 = GCO = -274.4 kJoule/mole
CoO entropy per mole O2 = SCoO = .12 kJoule/mole
CO entropy per mole O2 = SCO = .396 kJoule/mole
At the smelting temperature, the Gibbs energies of CoO and CO are equal and the
reaction is in equilibrium. Below this temperature oxygen binds to cobalt and
above this temperature it binds to carbon. The calculation is approximate
because it assumes entropy is a constant as a function of temperature. To
calculate the smelting temperature,
gCoO = gCO
GCoO - t SCoO = GCO - t SCO
t = (GCoO - GCO) / (SCoO - SCO)
= 558 Celsius
Tsmelt = 583 Celsius = t + 25 Celsius (The actual smelting temperature is 525 Celsius)
Gibbs Gibbs Entropy Entropy
kJoule/mole kJoule/mole(O2) kJoule/mole kJoule/mole(O2)
Li2O -561.9 -1123.8
Na2O -377 -754
K2O -322.2 -644.4
Cu2O -146.0 -292.0
Ag2O -11.2 -22.4
BeO -579.1 -1158.2
CO -137.2 -274.4 .198 .396
MgO -596.3 -1192.6 .0269 .0538
CaO -533.0 -1066.0 .0398 .0769
VO -404.2 -808.4
MnO -362.9 -725.8 .0597 .1194
CoO -214.2 -428.4
NiO -211.7 -423.4
CuO -129.7 -259.4 .0426 .0852
ZnO -318.2 -636.4
CdO -228.4 -456.8
HgO -58.5 -117.0
Fe3O4 -1014 -507 .0146 .0073
Co3O4 -795.0 -397.5
B2O3 -1184 -789
Al2O3 -1582.3 -1054.9 .0509 .0339
Ti2O3 -1448 -965.3
V2O3 -1139.3 -759.5
Cr2O3 -1053.1 -702.1 .0812 .0541
Fe2O3 -741.0 -494.0 .0874 .0583
CO2 -394.4 -394.4 .214 .214
SO2 .2481 .2481
SiO2 -856.4 -856.4 .0418 .0418
TiO2 -852.7 -852.7
MnO2 -465.2 -465.2 .0530 .0530
MoO2 -533.0 -533.0
WO2 -533.9 -533.9
PbO2 -219.0 -219.0
MoO3 -668.0 -445.3
WO3 -764.1 -509.4
V2O4 -1318.4 -659.2
Cu 0
C (gas) 672.8





 |
 |
_3.jpg) |
 |
 |
|---|---|---|---|---|
 |
 |
|---|---|
 |
 |
 |
 |
 |
|---|---|---|---|---|
 |
 |
|---|---|
 |
 |
 |
|---|---|---|
 |
 |
 |
 |
|---|---|---|---|
 |
 |
 |
 |
|---|---|---|---|
 |
 |
|---|---|
_.jpg) |
 |
|
|---|---|---|
.jpg) |
 |
 |
 |
|
|---|---|---|---|---|
 |
 |
 |
 |
 |
|---|---|---|---|---|
 |
 |
 |
|---|---|---|
 |
|---|
.jpg) |
 |
 |
 |
 |
|---|---|---|---|---|
 |
 |
 |
 |
|---|---|---|---|
 |
|---|