




 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
 |
 |
|---|---|---|
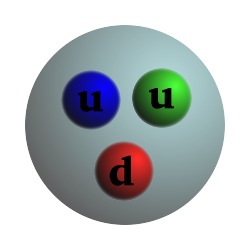
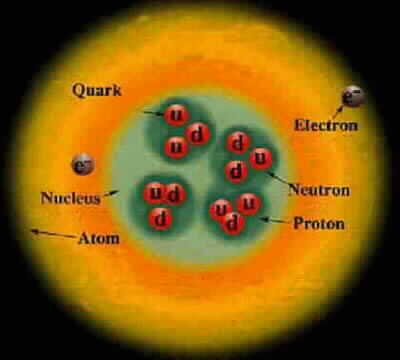
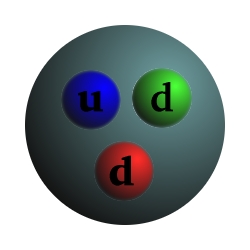
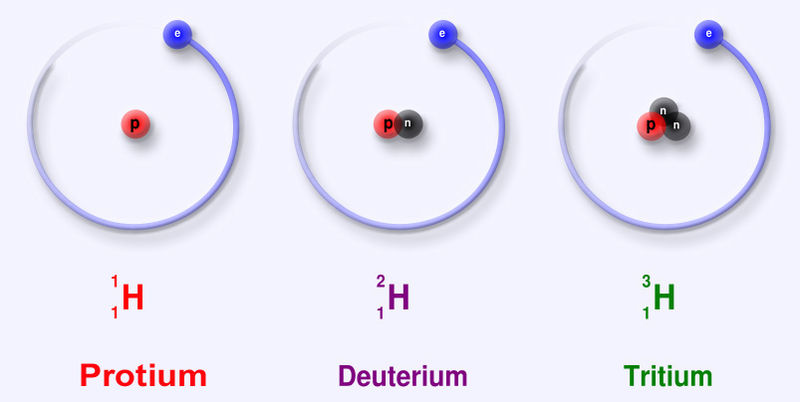
Particle Charge Mass Proton +1 1 Composed of 2 up quarks, 1 down quark, and gluons Neutron 0 1.0012 Composed of 1 up quark, 2 down quarks, and gluons Electron -1 .000544 Up quark +2/3 .0024 Down quark -1/3 .0048 Photon 0 0 Carries the electromagnetic force and binds electrons to the nucleus Gluon 0 0 Carries the strong force and binds quarks, protons, and neutronsCharge and mass are relative to the proton.
All of these particles are stable except for the neutron, which has a half life of 611 seconds.
Proton charge = 1.6022 Coulombs Proton mass = 1.673⋅10-27 kg Electron mass = 9.11⋅10-31 kg Hydrogen mass = Proton mass + Electron mass = 1.6739⋅10-27 kg
 |
|---|
An element has a fixed number of protons and a variable number of neutrons. Each neutron number corresponds to a different isotope. Naturally-occuring elements tend to be a mix of isotopes.
Isotope Protons Neutrons Natural fraction Hydrogen-1 1 0 .9998 Hydrogen-2 1 1 .0002 Helium-3 2 1 .000002 Helium-4 2 2 .999998 Lithium-6 3 3 .05 Lithium-7 3 4 .95 Beryllium-9 4 5 1 Boron-10 5 5 .20 Boron-11 5 6 .80 Carbon-12 6 6 .989 Carbon-13 6 7 .011Teaching simulation for isotopes at phet.colorado.edu
 |
 |
|---|---|
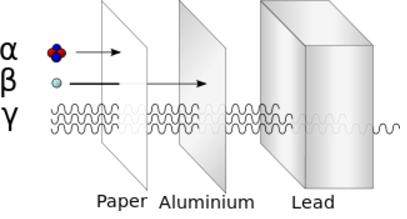
Alpha particle = Helium nucleus = 2 Protons and 2 Neutrons Beta particle = Electron Gamma ray = Photon Alpha decay: Uranium-235 -> Thorium-231 + Alpha Beta decay: Neutron -> Proton + Electron + Antineutrino (From the point of view of nuclei) Beta decay: Down quark -> Up quark + Electron + Antineutrino (From the point of view of quarks)Beta decay is an example of the "weak force".
For a radioactive material,
Time = T Half life = Th Original mass = M Mass remaining after time "T" = m = M exp(-T/Th)
Suppose an element has a half life of 2 years.
Time Mass of element remaining (kg) 0 1 2 1/2 4 1/4 6 1/8 8 1/16
 |
 |
|---|---|
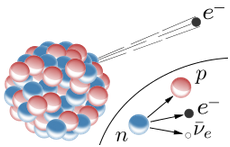
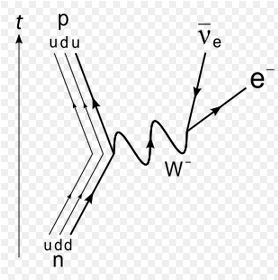
The weak force can convert a neutron into a proton, ejecting a high-energy electron.
From the point of view of nucleons: Neutron -> Proton + electron + antineutrino From the point of view of quarks: Down quark -> Up quark + electron + antineutrino
 |
 |
|---|---|
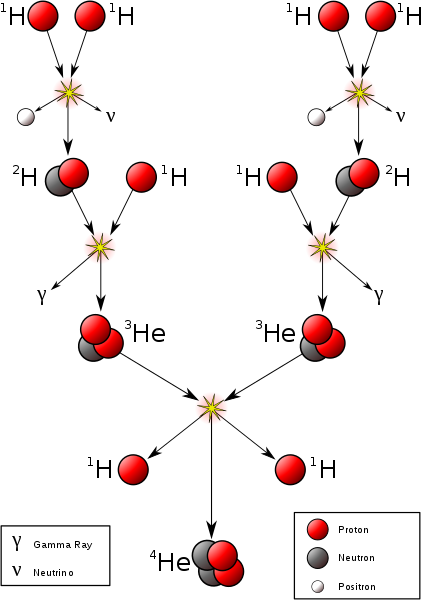
Hydrogen fusion requires a temperature of at least 4 million Kelvin, which requires an object with at least 0.08 solar masses. This is the minimum mass to be a star.
P + P → D + Positron + Neutrino + .42 MeV P + D → He3 + Photon + 5.49 MeV He3 + He3 → He4 + P + P + 12.86 MeV
 |
|---|
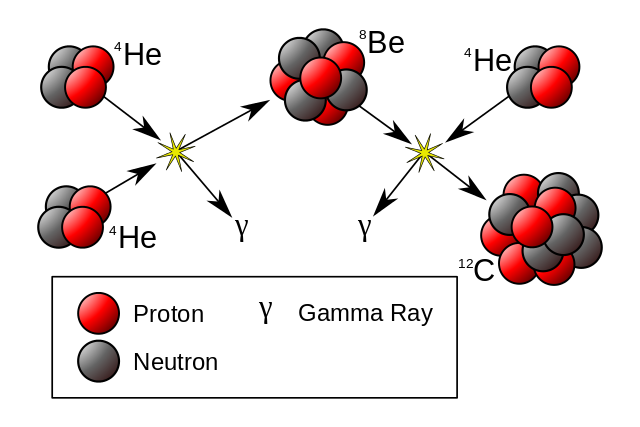
As the core of a star star runs out of hydrogen it contracts and heats, and helium fusion begins when the temperature reaches 10 million Kelvin
He4 + He4 -> Be8 - .092 MeV Be8 + He4 -> C12 + 7.367 MeV C12 + He4 -> O16 + Gamma + 7.162 MeV
 |
|---|
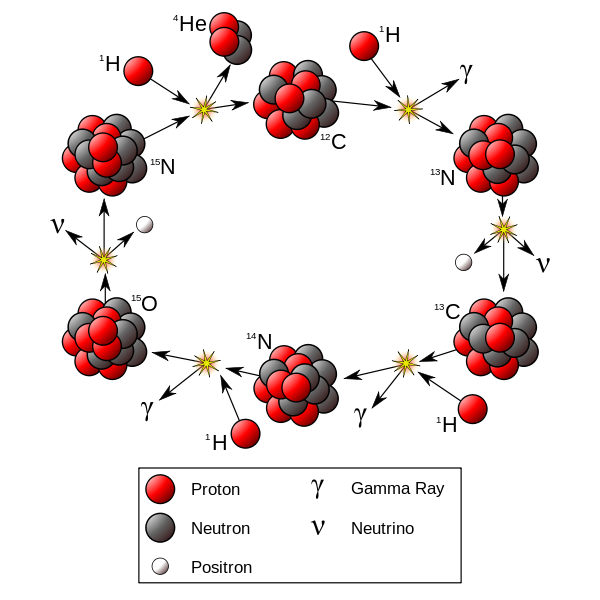
At temperatures above 17 million Kelvin, carbon-catalyzed fusion happens faster than proton-proton fusion. This occurs in stars more massive than 1.3 solar masses.
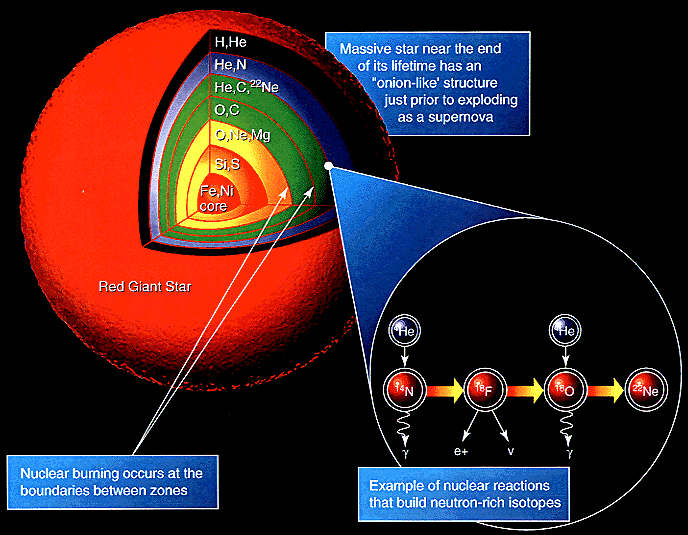 |
|---|
A heavy star continues to fuse elements until it reaches Iron-56. Beyond this, fusion absorbs energy rather than releasing it, triggering a runaway core collapse that fuses elements up to Uranium. If the star explodes as a supernova then these elements are ejected into interstellar space.
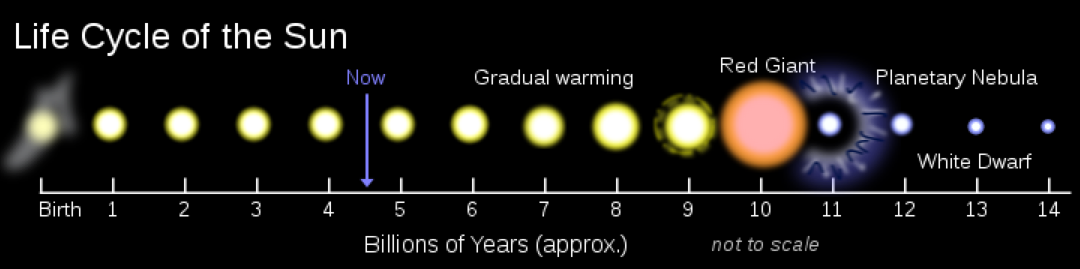 |
|---|
Star type Mass Luminosity Color Temp Lifetime Death Remnant Size of Output
(solar (solar (Kelvin) (billions remnant
masses) luminosities) of years)
Brown Dwarf <.08 1000 immortal
Red Dwarf .1 .0001 red 2000 1000 Red giant White dwarf Earth
The Sun 1 1 white 5500 10 Red giant White dwarf Earth Light elements
Blue star 10 10000 blue 10000 .01 Supernova Neutron star Manhattan Heavy elements
Blue giant 20 100000 blue 20000 .01 Supernova Black hole Central Park Heavy elements
Fate of stars, with mass in solar masses:
Mass < 9 → End as red giants and then turn white dwarf.
9 < Mass → End as supernova
9 < Mass < 20 → Remnant is a neutron star.
20 < Mass → Remnant is a black hole.
130 < Mass < 250 → Pair-instability supernova (if the star has low metallicity)
250 < Mass → Photodisintegration supernova, producing a black hole and relativistic jets.
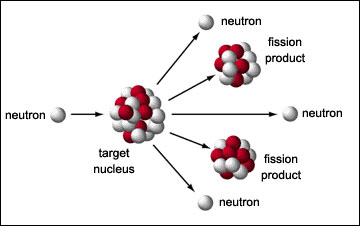 |
 |
|---|---|
A neutron triggers the fission of Uranium-235 and plutonium-239,
releasing energy and more neutrons. The released neutrons trigger further
fission.
 |
 |
 |
 |
 |
|---|---|---|---|---|
|
|
|
|
|
|
A fission of uranium-235 releases on average 1.86 neutrons, some of which trigger fission in nearby nuclei and some of which escape without triggering fission. If a sphere of uranium-235 is small then most of the neutrons escape before triggering fission and the sphere doesn't blow up. If the sphere is large then most of the neutrons trigger more fission, a chain reaction occurs and the sphere blows up. The threshold for a chain reaction is the "critical mass".
The nuclei that are capable of undergoing a chain reaction are:
Protons Neutrons Critical Halflife Neutrons per
mass (kg) (106 yr) fission
Uranium-233 92 141 16 .160 2.48
Uranium-235 92 143 52 700 1.86
Plutonium-239 94 145 10 .024 2.16
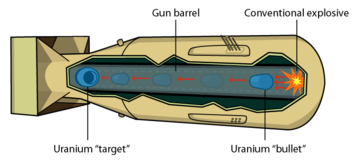 |
 |
|---|---|
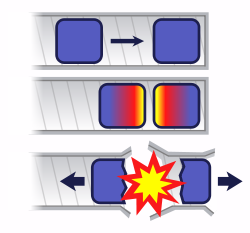
If you bring two pieces of uranium-235 together too slowly, a chain reaction begins in the near side of each piece, generates heat, and blows the two pieces apart before they can come completely together. Only a small amount of uranium undergoes fission and this is referred to as a "fizzle". Using gunpowder and a cannon is fast enough to properly detonate uranium and this is technologically easy to do.
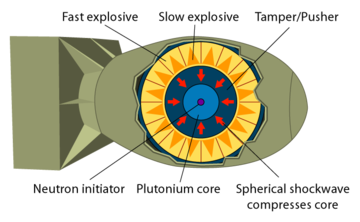 |
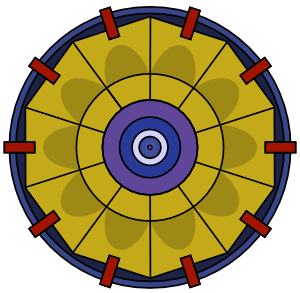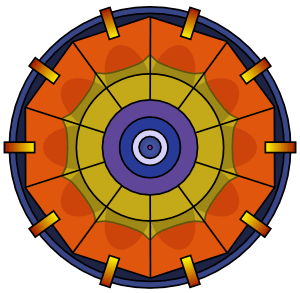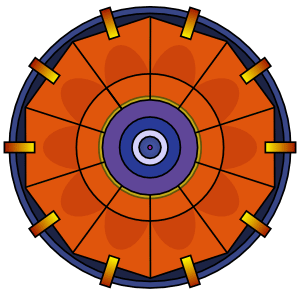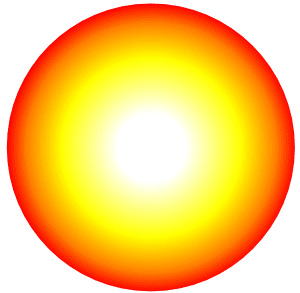 |
 |
 |
|---|---|---|---|
Plutonium is more difficult to detonate than uranium. Simply bringing two pieces together, no matter how fast, results in a fizzle. To detonate plutonium you have to shape it as a sphere and implode it, which is technologically difficult.
In World War 2 the U.S. produced enough uranium for 1 bomb and enough plutonium for 2 bombs. One of the plutonium bombs was tested in the "Trinity" test before being used in the war, and the second bomb was dropped on Nagasaki. The uranium bomb was dropped on Hiroshima without previously being tested.
When Hans Bethe, a physicist on the Manhattan project, was asked why they didn't test the uranium bomb he replied "Because we were perfectly sure it would work".
 |
 |
 |
|---|---|---|
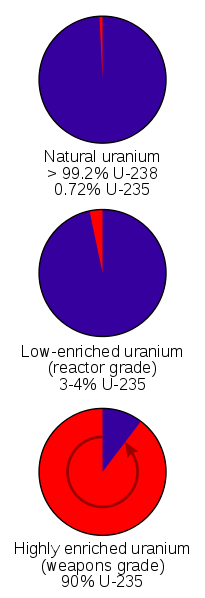
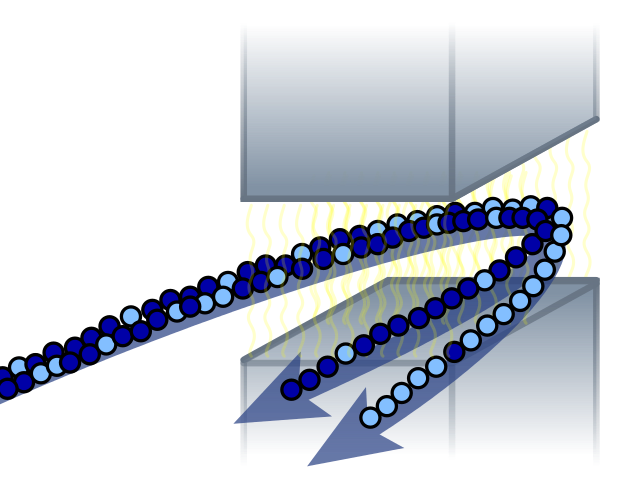
Natural Uranium is .72% Uranium-235 and 99.3% Uranium-238. Only Uranium-235 undergoes a chain reaction and so it has to be separated from the Uranium-238. Several methods exist for doing this. In World War 2 the isotopes were separated magnetically with calutrons. Gas diffusion and centrifuges can also be used.
 |
 |
 |
 |
|---|---|---|---|
Uranium is converted to gas form by forming uranium hexafluoride (HF6). HF6 is a gas above 64 Celsius. In a centrifuge, the lighter uranium-235 concentrates at the center and the heavier uranium-238 concentrates at the edge.
 |
|---|
Blue elements are unstable with a half life much less than the age of the solar system and don't exist in nature.
The only elements heavier than Bismuth that can be found on the Earth are Thorium and Uranium, and these are the only elements that can be tapped for fission energy.
Natural thorium is 100% Thorium-232
Natural uranium is .7% Uranium-235 and the rest is Uranium-238.
Plutonium has a short half life and doesn't exist in nature. It can be created by subjecting uranium-238 to neutrons in a nuclear reactor. Fissionable uranium-233 can be created from thorium-232.
Uranium-238 + Neutron → Plutonium-239 Thorium-232 + Neutron → Uranium-233 Detail: Uranium-238 + Neutron → Uranium-239 Uranium-239 → Neptunium-239 + Electron + Antineutrino Halflife = 23 minutes Neptunium-239 → Plutonium-239 + Electron + Antineutrino Halflife = 2.4 days Thorium-232 + Neutron → Thorium-233 Thorium-233 → Protactinium-233 + Electron + Antineutrino Halflife = 22 minutes Protactinium-233 → Uranium-233 + Electron + Antineutrino Halflife = 27.0 days
 |
|
|---|---|
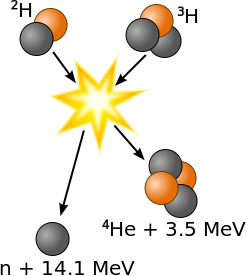
Fusion bombs use the reactions:
Neutron + Lithium6 → Tritium + Helium4 + 4.874 MeV Deuterium + Tritium → Helium4 + Neutron + 17.56 MeVLeaving out the neutron catalyst, this is
Deuterium + Lithium6 → Helium4 + Helium4 + 22.43 MeV
 |
 |
|---|---|
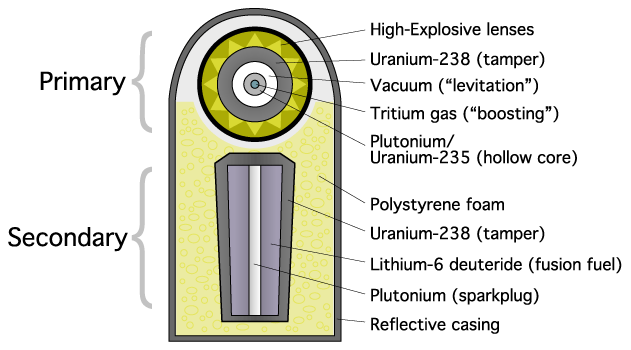
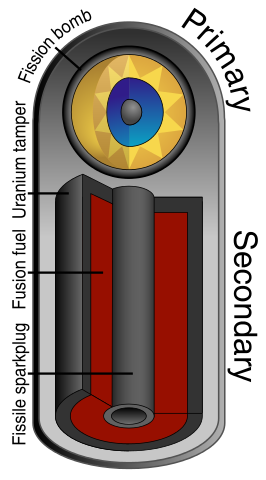
Fusion of deuterium and lithium requires high temperature and pressure, which is achieved by compressing the fuel. This is done by detonating a fission bomb and using the generated X-rays to compress the fusion fuel. X-rays strike the outer layer and expel atoms, and the recoil compresses the fuel. This is called "ablation" and the design was developed by Teller and Ulam.
X-ray Plasma Ablation
pressure pressure pressure
TPa TPa TPa
Ivy Mike 7.3 35 530
W-80 140 750 6400
-LLNL.jpg) |
 |
 |
|---|---|---|
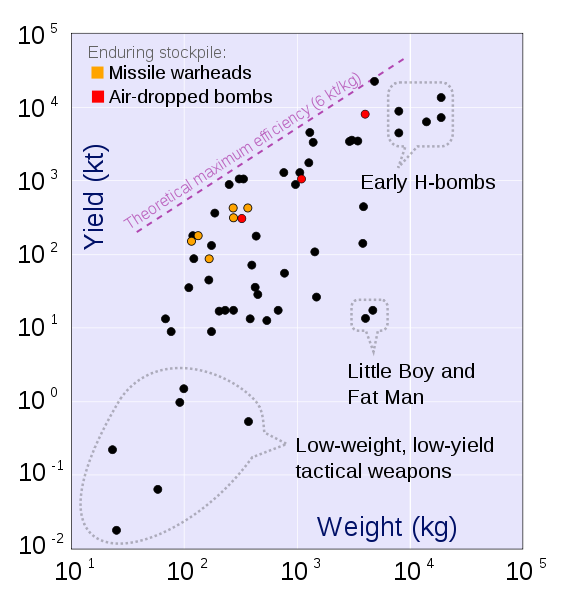 |
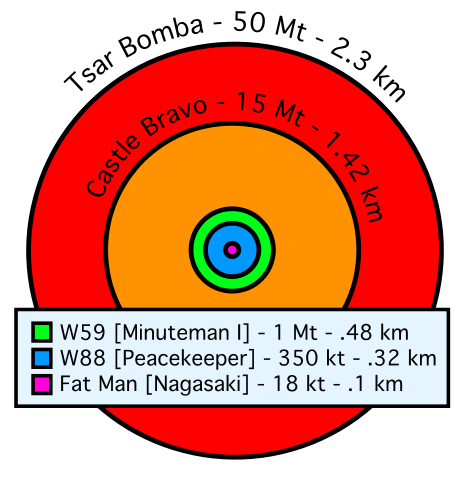 |
|---|---|
The practical limit for the energy/mass of a fusion bomb = 25 TJoules/kg or .0062 Mtons of TNT per kg.
1 ton of TNT = 4⋅109 Joules 1 ton of gasoline = 4⋅1010 Joules Massive Ordnance Air Blast bomb = .000011 MTons TNT (Largest U.S. conventional bomb) Trinity plutonium-239 test = .020 MTons TNT Hiroshima uranium-235 fission bomb = .015 MTons TNT "Little Boy". 60 kg Uranium-235 Nagasaki plutonium-239 fission bomb = .021 MTons TNT "Fat Man". 6 kg Plutonium-239 Ivy King fission bomb = .5 MTons TNT Largest pure fission bomb B83 fusion bomb = 1.2 MTons TNT Largest bomb in active service Castle Bravo fusion bomb = 15 MTons TNT Largest U.S. test B41 fusion bomb = 25 MTons TNT Largest U.S. bomb created Tsar Bomba = 50 MTons TNT Largest USSR test
 |
 |
 |
 |
|---|---|---|---|
 |
|---|
1885 Rontgen discovers X-rays
1899 Rutherford discovers alpha and beta rays
1903 Rutherford discovers gamma rays
1905 Einstein discovers that E=mc2. Matter is equivalent to energy
1909 Nucleus discovered by the Rutherford scattering experiment
1932 Neutron discovered
1933 Nuclear fission chain reaction envisioned by Szilard
1934 Fermi bombards uranium with neutrons and creates Plutonium
1938 Dec19 Hahn and Strassmann discover uranium fission
1939 Jan 6 Hahn and Strassmann publish uranium fission
1939 Jan25 Fermi begins conducting nuclear fission experiments at Columbia University
1939 Jan26 Bohr and Fermi report on uranium fission at the Washington Conference
on theoretical physics
1939 Szilard and Zinn discover that bombarding uranium with neutrons produces
new neutrons.
1939 Jul 4 Szilard, Wigner, and Einstein discuss nuclear fission
1939 Aug 2 Szilard, Teller, and Einstein discuss nuclear fission. Szilard drafts
the the "Einstein letter" that is later delivered to President Roosevelt
1939 Oct11 Alexander Sachs briefs President Roosevelt on Einstein's letter.
1939 Oct12 Alexander Sachs meets again with President Roosevelt and this time
Roosevelt gives the order to commence the development of a nuclear bomb.
1942 Dec 2 Fermi and Szilard achieve the first self-sustaining nuclear fission
reactor at the University of Chicago
1942 Aug Manhattan project commences
1942-1945 German nuclear bomb project goes nowhere
1945 Jul16 Trinity test of a plutonium bomb yields a 20 kTon TNT equivalent explosion
1945 Aug 6 A uranium bomb is deployed at Hiroshima, yielding 15 kTons TNT equivalent
1945 Aug 9 A plutonium bomb is deployed at Nagasaki, yielding 21 kTons TNT equivalent
Hans Bethe, a physicist on the Manhattan Project, was asked why the uranium type
bomb was not tested before deployment and he replied "Because we were perfectly sure
it would work".
 |
 |
 |
 |
 |
|---|---|---|---|---|
.jpg) |
 |
 |
|---|---|---|
All of the radioactive fission products decay by beta decay.
If the neutron cross section is 8 barnes or higher then the nucleus can potentially be transmuted into a nonradioactive nucleus.
Strontium-90 is ideal for Radioisotope Thermoelectric Generators (RTGs).
The most troublesome fission products are the ones that can't be transmuted. Chief among these are Caesium-137, Zirconium-93, Niobium-94, Strontium-90, Zirconium-91, and Palladium-107.
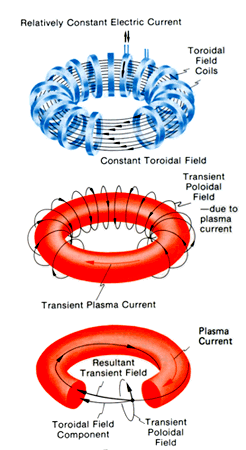
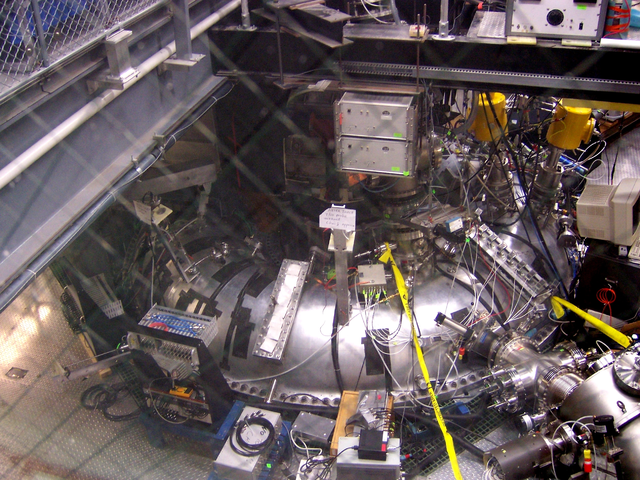
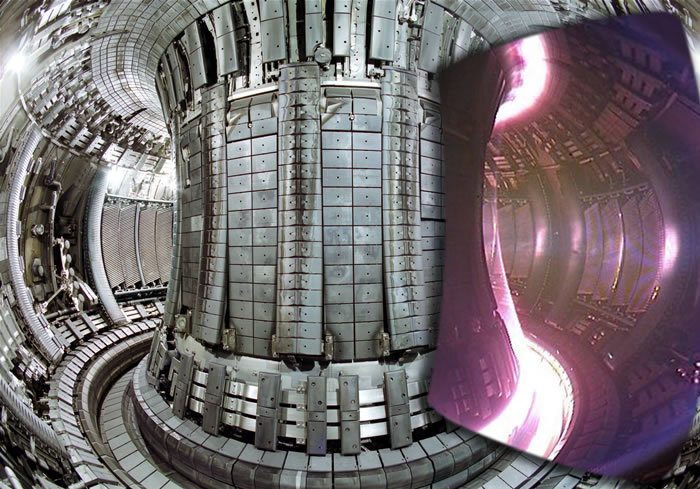
A tokamak confines a plasma
 |
|
|---|---|
Outer Lawson Magnetic Confine
radius field time
meter e20 Tesla second
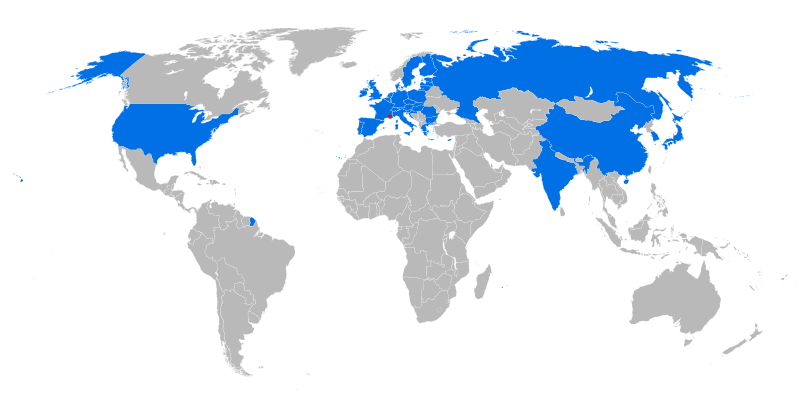
ITER 6.2 30 5.3 3000 France Future
ET 5.0 15 1.0 USA
JET 2.96 10 4.0 UK
JT-60 3.16 10 2.7 100 Japan
TFTR 2.4 9 6 Princeton
D III-D 1.66 4 2.2 San Diego
Alcator C .67 1 8 MIT
Tore Supra 2.25 .4 4.5 390 France
KSTAR 1.8 3.5 300 Korea
FTU .93 8 1.5 Italy
ASDEX 1.65 .12 3.9 10 Germany
A "*" denotes superconducting magnets.
 |
|
|---|---|
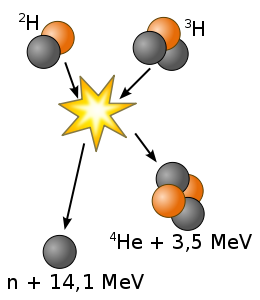
The easiest fusion reaction to achieve is
Deuterium + Tritium → Helium + Neutron
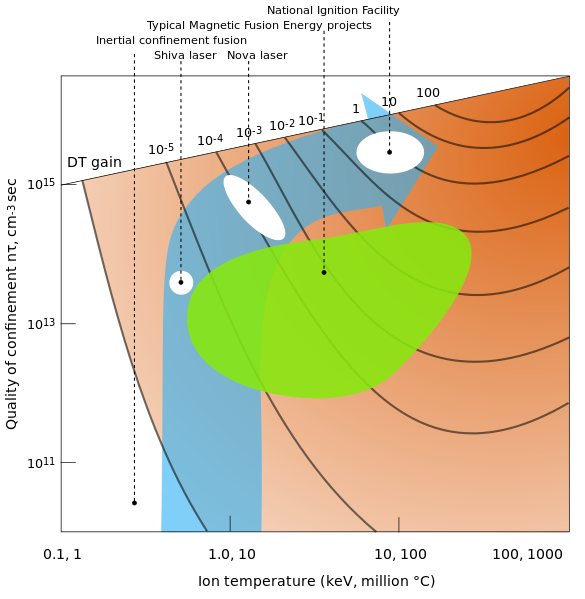
The optimal temperature is 500 million Kelvin and other fusion reactions require higher temperatures. This temperature can be achieved either with a tokamak, which confines a hot plasma with magnetic fields, or with "inertial confinement", where lasers compress and heat the material. The fuel density should be as high as possible to produce a satisfactory fusion rate.
 |
 |
|---|---|
 |
 |
 |
|---|---|---|
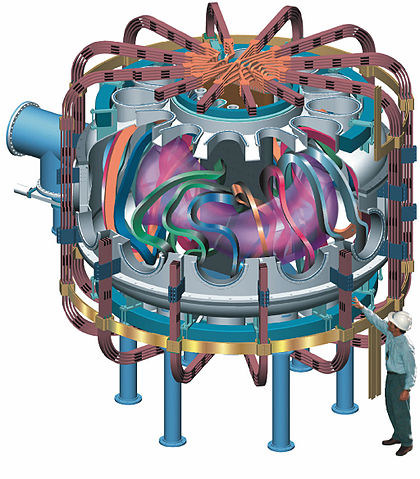
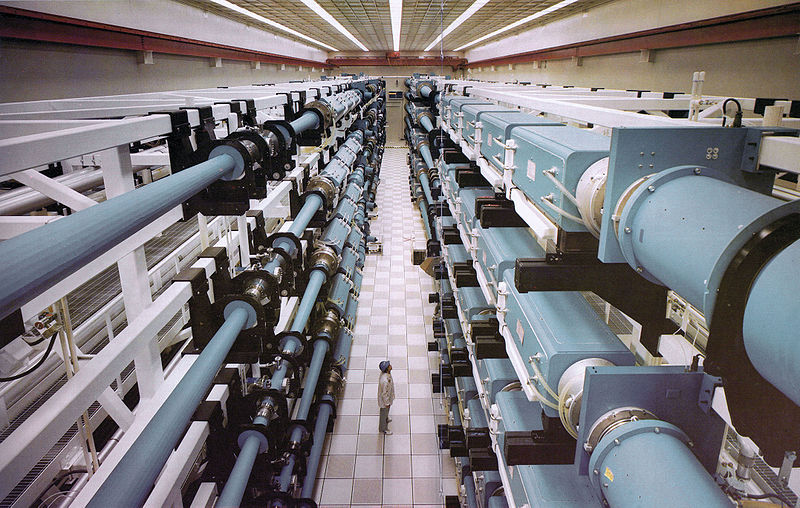
A tokamak uses magnetic fields to steer hot plasma around a donut-shaped vessel. The International Thermonuclear Experimental Reactor (ITER) in France is scheduled to begin operation in 2018. It will be the first tokamak that produces more fusion power than is required to operate the machine. There are numerous international participants and the experiment is as important for its superconducting magnet technology as it is for fusion. For the ITER reactor,
Fusion power = 500 MWatt Input power = 50 MWatt Temperature = 500 MKelvin Confinement time = 3000 seconds Plasma current = 17 MAmps Magnetic field = 5.3 Tesla Inner radius = 2.0 meter Outer radius = 6.0 meterThe "Lithium Tokamak Experiment" at the Princeton Plasma Physics Laboratory uses flowing liquid lithium walls to absorb hydrogen that escapes the plasma. This improves plasma confinement and is potentially a means for absorbing the heat generated by fusion neutrons.
 |
 |
|
|---|---|---|
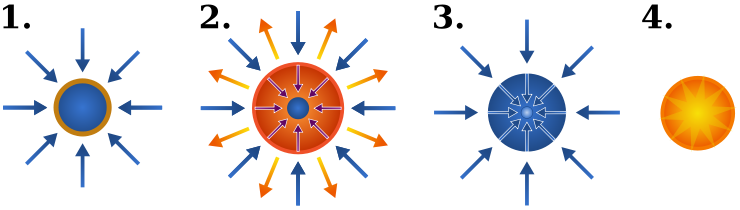
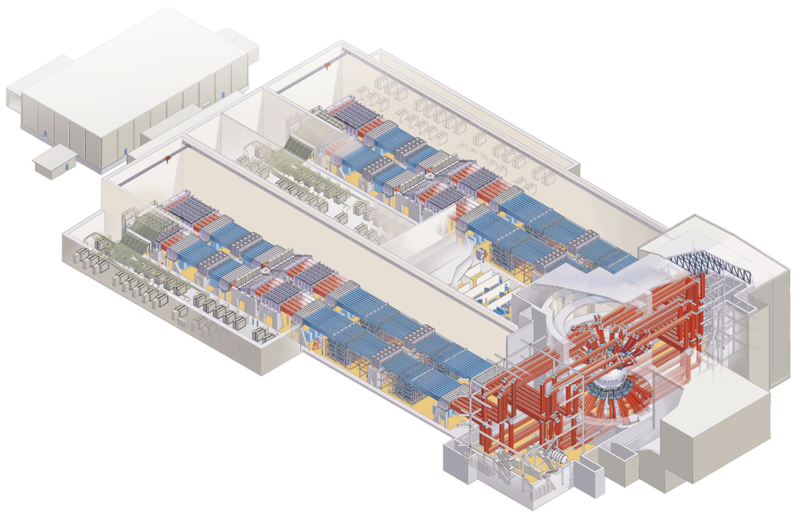
In laser fusion, lasers compress a sphere of fuel to a high enough density and temperature to fuse. The sphere consists of fusion fuel in the interior and an ablation layer as an outer shell. The laser ejects atoms from the ablation layer and the recoil compresses the fuel. The compression needs to be spherically symmetric and so a large number of lasers are used to evenly distribute the energy over the sphere.
The National Ignition Facility uses one laser pulse and it found that this is not able to achieve break-even fusion energy. The HiPer experiment was subsequently built to test a two-pulse strategy. The first pulse compresses the fuel and a second pulse further heats it. The first pulse is spherically symmetric and the second pulse is one beam fired into the core of the compression zone.
Electric Laser Fusion Target
energy energy energy density
MJ MJ MJ g/cm3
National Ignition Facility 330 1.85 20 1000 1 laser pulse
HiPer 422 .27 25 300 2 laser pulses
Electric energy Energy supplied to the system
Laser energy Laser energy delivered to the target
Fusion energy Energy produced by fusion of the traget
Target density Density of the target after laser compression
 |
 |
|
|---|---|---|
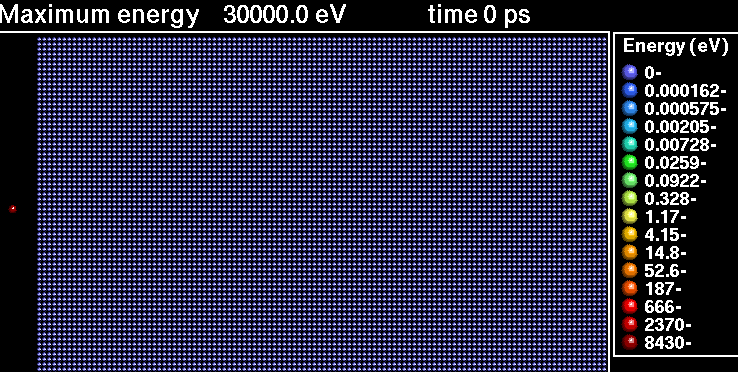
The fusion of deuterium and tritium produces neutrons with an energy of 14.1 MeV. These neutrons dislodge atoms in materials, weaking the material.
In the following sequence of frames a 30 keV Xenon ion crashes into gold, disrupting the positions of atoms.
 |
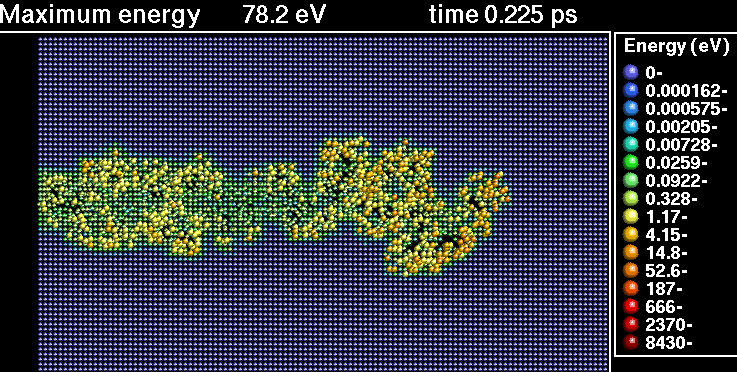 |
|---|---|
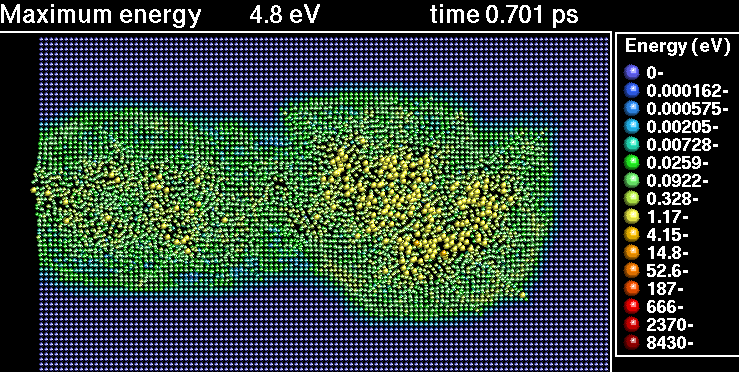 |
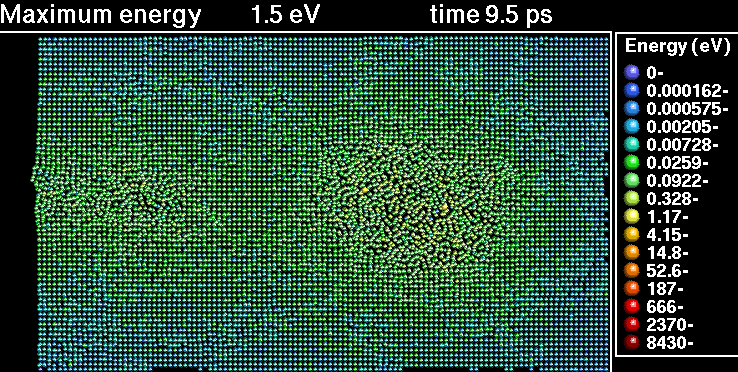 |
|---|---|
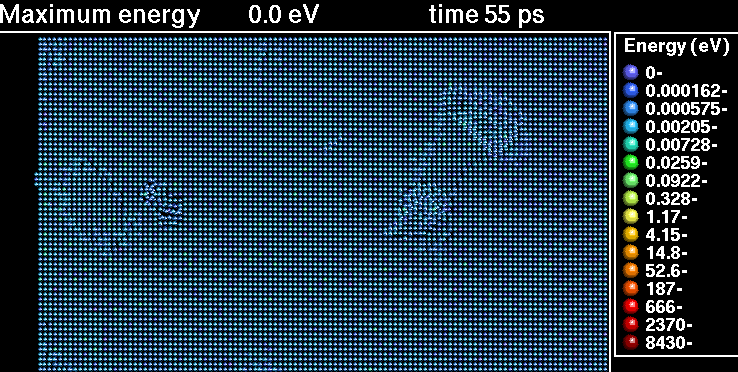 |
|
|---|---|
Liquid lithium wallls are being considered for stopping the neutrons. Lithium also absorbes hydrogen that escapes the plasma and improves the plasma confinement properties.
Lithium Tokamak Experiment
International Fusion Materials Irradiation Facility
The fusion reactions that don't produce neutrons are
Energy Coulomb Aneutronic
yield energy
MeV
P + P → D + e+ .42 1 * Slow because it needs the weak force
P + D → He3 + g 5.49 1 * Slow because it needs the electromagnetic force
D + D → T + P 4.03 1 * 50%. Tritium = 1.01 MeV, Proton = 3.02 MeV
→ He3 + N 3.27 1 50%. He3 = .82 MeV, Neutron = 2.45 MeV
D + T → He3 + N 17.58 1 He3 = 3.52 MeV, Neutron = 14.06 MeV
T + T → He4 + 2N 11.3 1
He3 + D → He4 + P 18.353 2 * D+D side reactions make neutrons
He3 + T → He4 + P + N 12.1 2 57%
→ He4 + D 14.3 2 * 43%
He3 + He3 → He4 + P + P 12.860 4 *
Li6 + P → He4 + He3 4.0 3 *
Li7 + P → He4 + He4 17.2 3 *
Li6 + D → He4 + He4 22.4 3 * D+D side reactions produce neutrons
→ He4 + He3 + N 2.56 3
→ Li7 + P 5.0 3 *
→ Be7 + N 3.4 3
Li6 + He3 → He4 + He4 + P 16.9 6 *
B11 + P → He4 + He4 + He4 8.7 5 *
N15 + P → C12 + He4 5.0 7 *
C13 + He4 → O16 + N Stellar s-process
Ne22 + He4 → Mg25 + N Stellar s-process
Li6 + N → He4 + T 4.784 *
Li7 + N → He4 + T + N -2.467
"Coulomb energy" is the product of the charges of the two reactants, in units
of proton charge. The lower the energy, the easier it is to fuse the nuclei.
This can be seen with
the Rutherford
scattering simulation.Helium-3 is rare on the Earth and abundant on the moon.
n = Electron density
M = Electron mass
V = Electron thermal velocity
Q = Proton charge
k = Boltzmann constant
Τ = Temperature
T = Confinement time
I = Plasma current
B = Magnetic field in Teslas
Xdebye = Debye length (k Τ/n/Q2/(4 π Ke))½
Xgyro = Electron gyro radius M V / Q B
Fgyro = Electron gyrofrequency
Electron Temp Debye Magnetic
density (K) (m) field (T)
(m-3)
Solar core e32 e7 e-11 -
ITER 1.0e20 e8 e-4 5.3
Laser fusion 6.0e32 e8 - National Ignition Facility. Density=1000 g/cm3
Gas discharge e16 e4 e-4 -
Ionosphere e12 e3 e-3 e-5
Magnetosphere e7 e7 e2 e-8
Solar wind e6 e5 e1 e-9
Interstellar e5 e4 e1 e-10
Intergalactic e0 e6 e5 -
A rocket ideally produces as much energy per mass as possible, which is reflected in the fusion "energy per nucleon". The reactions that have the best energy per nucleon are
Energy Energy per
yield nucleon
(MeV) (MeV)
D + He3 -> He4 + P 18.353 3.67
D + T -> He4 + N 17.590 3.52
D + Li6 -> He4 + He4 22.4 2.80
T + He3 -> He4 + D 14.320 2.39 41%
P + Li7 -> He4 + He4 17.2 2.15
He3 + He3 -> He4 + P + P 12.860 2.14
T + He3 -> He4 + P + N 12.096 2.02 59%
The best choice is D + He3 and the next best choice is D + T.
Energy Energy per
yield nucleon
(MeV) (MeV)
P + P -> D + Positron .42 .21
P + D -> He3 + Photon 5.49 1.83
P + T -> He3 + N -.764
P + Li6 -> He4 + He3 4.0 .57
P + Li7 -> He4 + He4 17.2 2.15
P + B11 -> He4 + He4 + He4 8.7 .72
D + D -> T + P 4.033 1.01 50%
-> He3 + N 3.269 .81 50%
D + T -> He4 + N 17.590 3.52
D + He3 -> He4 + P 18.353 3.67
D + Li6 -> He4 + He4 22.4 2.80
T + T -> He4 + N + N 11.332 1.89
T + He3 -> He4 + P + N 12.096 2.02 59%
-> He4 + D 14.320 2.39 41%
He3 + He3 -> He4 + P + P 12.860 2.14
He3 + Li6 -> He4 + He4 + P 16.9 1.88
N + Li6 -> T + He4 4.784 .68
N + Li7 -> T + He4 + N -2.467
Mass of nucleus Mass of atom Half life Binding energy
(AMU) (AMU) per nucleon (MeV)
Electron .00054858
Neutron 1.00866492 886 seconds 0
Proton 1.00727647 0
Hydrogen 1.00727647 1.00782504 0
Deuterium 2.01355321 2.01410178 1.11226
Tritium 3.01550071 3.01604928 12.3 years 2.82727
Helium-3 3.01493173 3.0160293 2.57269
Helium-4 4.00150485 4.002602 7.07392
Lithium-6 6.01347537 6.01512280 5.33257
Lithium-7 7.01435712 7.01600455 5.60637
Beryllium-8 7*10^17 s 7.06244
Beryllium-9 6.46278
Boron-10 6.47508
Boron-11 6.92771
Carbon-12 7.68015
Carbon-13 7.46986
Carbon-14 5730 years 7.52033
Oxygen-16 7.97622
Oxygen-17 7.75075
Oxygen-18 7.76707
Iron-56 8.79
Uranium-235 7.59
Uranium-238 7.57
1 MeV = 106 eV = 1.602*10-13 Joules
If no half-life is given, the nucleus is stable
1 atomic mass unit (AMU) = 1.660538921*10-27 kg = 931.494061 MeV
Cross section (barns)
Magnesium .059
Lead .17
Zirconium .18
Aluminum .23
Iron 2.56
Stainless 3.1
Nickel 4.5
Titanium 6.1
Cadmium 2520
A fission chain reaction requires that the material produces on average more than one neutron upon fission. The materials that qualify are:
Nucleons Critical Critical Fission Neutrons Decay Fission Fission
mass diameter area /fission halflife halflife rate
kg cm barns Myear Tyear F/s/kg
Californium 252 2.73 6.9 2.32 3.73 .0000026
Californium 251 5 8.5 2.43 .000290 -
Californium 249 6 9 1.74 .000351
Neptunium 236 6.79 8.7 .154 Inf 0
Curium 247 7.0 9.9 15.6
Curium 243 8 10.5 .000029
Plutonium 238 9.5 9.7 1.99 .000088 1204000
Plutonium 239 10 9.9 1.80 2.16 .024 5500 10.1
Curium 245 10 11.5 .0085
Americium 242 11 12 .000141
Plutonium 241 12 10.5 1.65 .000014 <.8
Uranium 233 15 11 1.95 2.48 .159 -
Plutonium 240 40 15 1.36 2.21 .0066 .116 478000
Uranium 235 52 17 1.24 1.86 704 350000 .0056
Neptunium 237 60 18 1.34 2.14 <.05
Americium 241 83.5 1.38 .00043 500
Plutonium 242 95 1.13 805000
Protactinium 231 >188 .83 <5
Uranium 238 Inf Inf .31 2.07 4470 8400 5.51
Curium 250 Inf Inf 3.31 .0069
Thorium 232 Inf .08 <.00005
Uranium 232 >5 2.01 .002
Uranium 234 >41 1.22 3.9
Uranium 236 >167 .59 2.30
Protactinium 233 .46
Americium 243 1.10
Fission area = Fission cross section in barns (10-28 meters2)
Californium-252
Californium-249
Data
Data
# of Fission Fusion
bombs
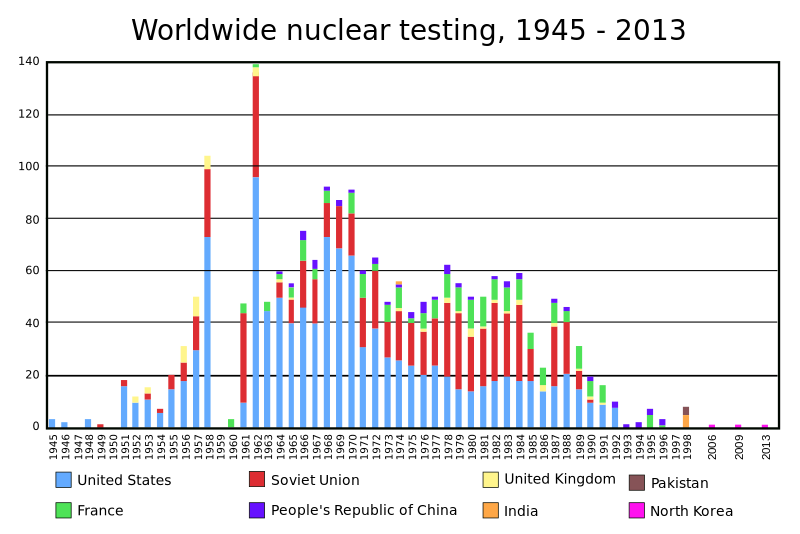
USA 7260 1945 1954
Russia 7500 1949 1954
France 300 1960 1968
China 260 1964 1967
UK 215 1952 1957
India 110 1974 Uranium fission
Israel 80 1979 Unknown
S. Africa 0 1980 Dismantled in 1991
Pakistan 120 1998 Uranium fission. >1500 kg of uranium-235, 20 kg per bomb
N. Korea 20 2009 Plutonium fission
Germany 1944 Attempted fission and failed
Iran 1981 Osirak reactor to create Plutonium. Reactor destroyed by Israel
Pakistan 1990 Commenced building centrifuges to enrich uranium from stolen designs.
Bomb tersted in 1998.
Iraq 1993 Attempted magnetic enrichment of Uranium. Dismantled after Gulf War 1
Iraq 2003 Alleged by the United States. Proved to be untrue.
N. Korea 2006 Created plutonium in a nuclear reactor. Detonation test fizzled.
Also acquired centrifuges from Pakistan
Also attempting to purify Uranium with centrifuges
Syria 2007 Nuclear reactor destroyed by Israel
Iran 2009 Attempting centrifuge enrichment of Uranium.
Libya -- Attempted centrifuge enrichment of Uranium. Dismantled before
completion. Cooperated in the investigation that identified
Pakistan as the proliferator of Centrifuge designs.
Libya 2010 Squabbling over nuclear material
Libya 2011 Civil war
North Korea has enough plutonium for an estimated 20 fission bombs.
2006 plutonium test = .001 Mtons 2009 plutonium test = .005 Mtons 2013 plutonium test = .010 Mtons 2016 plutonium test Jan 6 = .010 Mtons 2016 plutonium test Sep 6 = .010 Mtons
 |
 |
 |
|---|---|---|
 |
 |
_shock_trials.jpg) |
|---|---|---|
 |
 |
 |
|---|---|---|
 |
 |
 |
|---|---|---|
 |
 |
|---|---|
 |
 |
.jpg) |
 |
.jpg) |
|---|---|---|---|---|
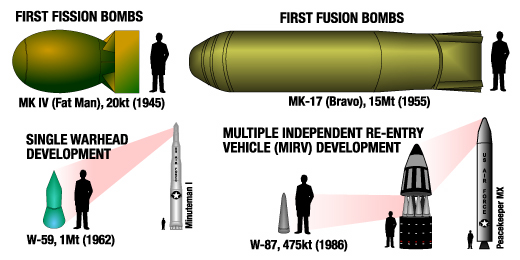
Yield Mass Mton/ Fission # Start End Platform
Mton kg kg primary built
B41 25 4850 5.15 500 1961 1976 B-52, B-47 Succeeded by the B53
B53 8.9 4010 2.22 340 1962 1997 Titan II Bunker buster
W56 1.2 272 4.96 1963 1993 Minuteman
B83 1.2 1100 1.09 650 1983 Current Bomber
W88 .48 <360 1.33 Komodo Current Trident
W87 .48 ~235 2.04 1986 Current Minuteman
W78 .35 ~340 1.03 1979 Current Minuteman
B61 .34 320 1.06 B61 3155 1968 Current Bomber Bunker buster. Tunable to .3 kilotons
W80 .15 130 1.15 B61 2117 1984 Current Tomahawk Tunable to 5 kiloton
W84 .15 176 .85 B61 530 1983 Current Tomahawk Tunable to .2 kilotons
W76 .10 164 .61 >2000 1978 Current Trident
Tzar Bomba 50 27000 1.85 1 1961 1961
The B41 and Tzar Bomba are three-stage devices (fission-fusion-fusion).
 |
 |
 |
|---|---|---|
Fission Fusion primary secondaries RACER IV Mark 14, Mark 16, Mark 17 Python B28, W28, W40, W49 Boa W30, W52 Robin W38, W45, W47 Tsetse W43, W44, W50, B57, W59 Kinglet W55, W58 B61 B61, W69, W73, W80, W81, W84, W85, W86
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
|---|
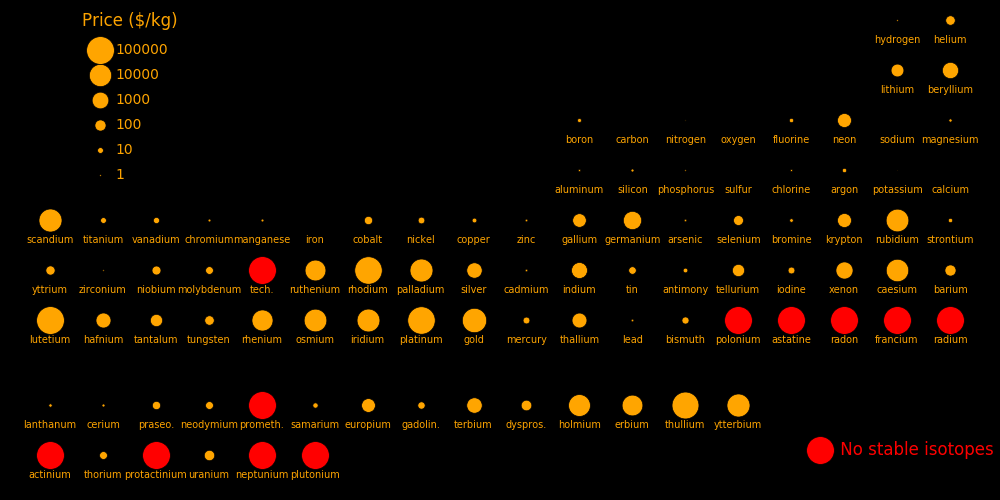
The neutrons in a nuclear reactor can transmute elements. Transmutation increases the proton number of an element by one. Profitable transmutations are locations in the periodic table where a high-value element is just to the right of a low-value element. The most profitable elements that can be created are:
Protons Price Transmutation
$/kg rate in barns
71 Lutetium 100000 2.0
45 Rhodium 88000 1.01
78 Platinum 88000 425
69 Thullium 70000 3.2
70 Ytterbium 14000
77 Iridium 13000 33
76 Osmium 12000 90
67 Holmium 8600 887
75 Rhenium 6200 11.4
68 Erbium 5400 65
54 Xenon 1200 6.15
Elements with no stable isotope can be created, such as technetium, polonium, radium, protactinium, and all the elements beyond uranium.
An example of a transmutation is:
Dysprosium-164 + Neutron -> Dysprosium-165 Neutron captures Dysprosium-165 -> Holmium-165 + Electron Beta decay with a half life of 2.3 hours.
The transmutation rate is proporational to the neutron capture cross section, measured in "barns" (10-28 meters2). The fastest transmutations are holmium and iridium.
Burnt fission fuel contains valuable elements. One 1 kg of burnt uranium-235 produces $2700 of rhodium, $290 of ruthenium, and $200 of xenon. Xenon is easily extracted because it's a gas. The valuable elements produced by Uranium-235 are:
Fraction Value of element Value in burnt fuel
$ per kg of element $ per kg of burnt fuel
Rhodium .0304 88000 2700
Ruthenium .0517 5600 290
Xenon .1683 1200 202
The valuable elements produced by plutonium-239 fission are:
Fraction Value of element Value in burnt fuel
$ per kg of element $ per kg of burnt fuel
Rhodium .03474 88000 3060
Palladium .04188 13600 570
Xenon .04410 1200 53
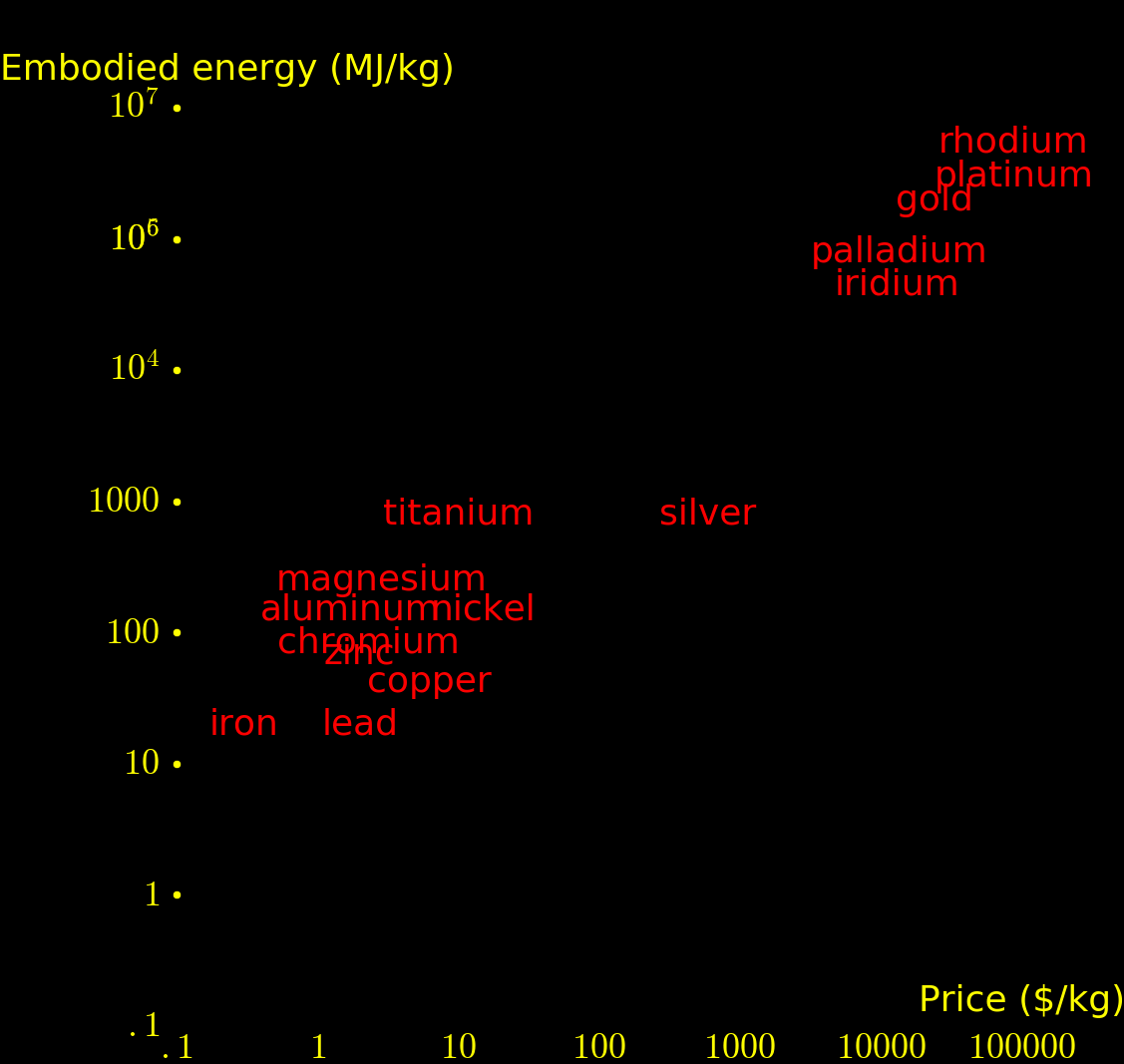 |
|---|
For most elements, production cost is dominated by energy. The above plot shows the energy required to produce each metal.
Fission energy/mass = e =85000000 MJoules/kg Reactor efficiency = f = .33 Price of energy = S = 36 MJoules/$ $ yield per kg = Y = fe/S = 790000 $/kg
If neutrons are used for fission energy then they produce 790000 $ per kg of uranium. If the neutrons are instead used to transmute elements then the highest-value elements that can be created have a value in the range of 50000 $/kg.
 |
|---|
Isotope with the longest half-life.
Technetium 98 4200000 Promethium 145 17.7 Polonium 209 125.2 Astatine 210 .00092 Radon 222 .0105 Francium 223 .000042 Radium 226 1600 Actinium 227 21.8 Thorium 232 14056000000 Protactin 231 32760 Uranium 238 4468000000 Neptunium 237 2140000 Plutonium 244 80000000 Americium 243 7370 Curium 247 15600000 Berkelium 247 1380 California 251 898 Einstein 252 1.29 Fermium 257 .28 Mendelev 258 .14
Isotopes with long half lives:
Half life
(years)
Hydrogen 3 12.3
Cobalt 60 5.27
Technetium 97 2600000
98 4200000
99 211100
Strontium 90 28.8
Radium 226 1600
Thorium 232 14056000000
Protactin 231 32760
Uranium 232 69
233 159200
234 245500
235 703800000
236 23400000
238 4468000000
Neptunium 236 154000
237 2140000
Plutonium 238 87.7
239 24110
240 6563
241 14
242 272300
244 80000000
Americium 241 432
243 7370
Curium 243 29.1
244 18.1
245 8500
246 4730
247 15600000
248 340000
250 9000
Berkelium 247 1380
249 .90
California 248 .91
249 351
250 13.1
251 898
252 2.64
253 .049
254 .17
Einstein 252 1.29
254 .75
Einstein 252 1.29
254 .75
Fermium 257 .28
Mendelev 258 .14
Natural Thermal Half life Decay Fission Thermal
fraction neutron mode neutron
capture scatter
barns barns barns
Hydrogen 1 .99985 .3326 20
2 .00015 .000519 4
3 0 0
Helium 3 5333
4 0
Lithium 6 940
7 .0454
Boron 10 200 2
Carbon All .002 5
Oxygen 16 .0001 4
Calcium 40 .969 .41
43 .00135 6.2
44 .021 .88
45 0 163 days Beta
Chromium 52 .5 3
Iron 55 2.737 years EC
56 2 10
Cobalt 56 .211 years Beta+
57 .744 years EC
58 .194 years Beta+
59 1 37.2 6
60 0 5.27 years Beta, gamma
Nickel 58 3 20
Scadium 45 1
Gallium 69 .601 2.18
70 0 21 min Beta
71 .399 3.61
72 0 14.1 hours Beta
Germanium 70 .205
72 .274
Selenium 80 .498
Bromine 85 0 2.9 min Beta
Krypton 78 .0035 6.4
80 .022 11.8
82 .116 29
83 .115 185
84 .57 .113
85 0 11 years Beta
86 .173 .003
Rubidium 85 .722
85m 0 4.48 hours Beta More complication
86 0 18.7 days Beta
87 .278
88 0 18 min Beta
Strontium 90
Zirconium 90 .514 .006 5
91 .112
92 .172
93 0 1.53 Myears Beta
94 .174
95 0 64 days Beta
96 .0280
97 0 16.7 hours Beta
Niobium 93 1
94 0 20300 years Beta
95 0 35 days Beta
96 0 24 hours Beta
97 72 min Beta
Molybdenum 92 .146
93 0 4.00 MYear Electron capture
94 .092
95 .159 13.4
96 .167 .5
97 .096 2.2
98 .243 .13
99 0 2.75 days Beta
100 .097 .199
101 0 15 minutes Beta
Technetium 96 4.3 days Positron
97 4.2 MYear Electron capture
98 4.2 MYear Beta
99 22.8 211000 years Beta
100 16 seconds Beta
101 14 min Beta
Ruthenium 96 .0554
97 2.79 days Positron
98 .0187
99 .128
100 .126 4.8
101 .17 3.3
102 .316 1.17
103 0 1.2 39 days Beta
104 .187 .31
105 0 4.44 hours Beta
106 0 .146 374 days Beta
107 0 3.75 min Beta
Rhodium 103 1
104 0 42.3 seconds Beta 99.55%, Positron otherwise
105 0 35.36 hours Beta, gamma
106 0 29.8 seconds Beta
Palladium 106 .2733
Cadmium 113 .122 30000 100
114 .288
Indium 115 100 2
Tin 122 .0463
125
127 2.1 hours
Antimony 122 2.72 days Beta 98%
123 .428
124 60.2 days Beta
125 2.76 years Beta
126 12.4 days Beta
127 3.8 days Beta
Tellurium
127 9.4 hours
132 3.20 days
Iodine 127 1 6.15
128 0 25 minutes Beta 93%
129 0 15.7 Myear Beta
131 0 8.02 days Beta, gamma
132 0 2.30 hours Beta
133 0 20.8 hours Beta
134 0 52.5 min Beta
135 0 6.57 hours Beta
Xenon 124 .001 165
125 0 17 hours Positron
126 .0009 3.5
127 0 36.3 days Electron capture
128 .0191 8
129 .264 21
130 .041 26
131 .212 85
132 .269 .45
133 0 5.2 days Beta
133m 0 2.19 days Internal
134 .104 .265
135 0 2000000 9.1 hours Beta 400000
135m 0 15.3 min Internal
136 .089 .26
Caesium 133 1 30.3
134 0 2.06 years Beta and Electron capture
135 0 2.3 Myears Beta
136
137 0 30.2 years Beta
Barium 134
140 0 12.8 days Beta
Lanthanum 138 .00089 1.05e11 years Beta and electron capture
139 .99911
140 0 1.68 days Beta
141 0 3.92 hours Beta
Cerium 140 .884 .57
141 0 32.5 days Beta
142 .111
143 0 33.0 days Beta
144 0 285 days Beta
Praseodym 141 1
144 17.3 min Beta
151 18.9 second Beta
Neodymium 142 .272 18.7
143 .122 337
144 .238 3.6
145 .0829 42
146 .172 1.4
147 0 11 days Beta
148 .0575 2.5
149 0 1.7 hours Beta
150 .0563 1.2
151 0 12.4 min Beta
Promethium 145 0 17.7 years Electron capture
146 0 5.5 years Electron capture
147 0 2.6 years Beta
149 0 53.1 hours Beta
151 0 28.4 hours Beta
Dysprosium 156 .00056 33
158 .00095 43
160 .0233 56
161 .189 600
162 .255 194
163 .249 124
164 .282 2840
165 0 139 min
Samarium 144 .0308
147 .150 57
148 .112
149 .138 42080
150 .0737
151 0 90 years Beta
152 .267
153 0 46.28 hours Beta
154 .227
Europium 151 .478 9100
152 0 13.5 years Beta and Electron capture
153 .522 312
154 8.59 years Beta
155 4.76 years Beta
156 15.2 days Beta
Gadolinium 155 .148
156 .205
Terbium
Dysprosium 156 .00056
157 0
158 .00095
159 0
160 .0233
161 .189
162 .254
163 .249
164 .283
165 2.33 hours Beta
Holmium 165 1 65
166 27.3 hours
Erbium 166 .335 19.6
167 .229 659
168 .271 2.74
169 0
170 .149 5.8
Thulium 169 1 100
170 129 days Beta
Ytterbium 168 .0014 2230
170 .031 11.4
171 .143 48.6
172 .219 .8
173 .161 17.1
174 .318 69.4
175 4.18 days Beta
176 .127 2.85
177 0 1.9 hours Beta
Lutetium 175 .974 21
176 .0261 2065
177 6.6 days Beta
Tungsten 180 .0012 60
181 0 121 days Electron capture
182 .265 20.7
183 .143 10.1
184 .307 2.0
185 0 75 days Beta
186 .286 35
187 0 23.7 hours Beta
Rhenium 185 .374 112
186 0 3.72 days Beta
187 .626 76.4
188 0 17.0 hours Beta
Osmium 186 .0158 80
187 .016 320
188 .133 4.7
189 .161 25
190 .264 13.1
191 0 15.4 days Beta
192 .41 2
193 0 30.1 hours Beta
Iridium 191 .373 954
192 0 73.8 days Beta
193 .627 111
194 0 19.3 hours Beta
Platinum 190 .0001 10.3
192 .0079 10.0
193 50 years Electron capture
194 .329 1.44
195 .338 27.5
196 .253 .72
197 19.9 hours Beta
198 .072 3.66
199 30.8 minutes Beta
Gold 197 1 98.6 8.2
198 1.70 days Beta
Lead 204 .014
205 -
206 .241
207 .221
208 .524
209 - 3.25 hours Beta
210 - 22.3 years Beta
Bismuth 209 1 .0338
210 5.01 days Beta
Polonium 208 2.898 years Alpha or Beta+
209 125.2 years Alpha or Beta+
210 .379 years Alpha
Natural Thermal Half life Decay Fission Critical
fraction neutron mode barns mass (kg)
capture
(barns) years
Thorium 232 .9998 7.56
233 0 .000042 Beta
Protactin 231 0 210 33000 Alpha
233 0 41? .074 Beta
Uranium 233 0 73 159200 468 15
234 .00005 105 245500
235 .0072 690 704000000 538 52 Thermal neutron scatter = 10 barns
236 0 7 23400000
237 .018
238 .993 2.68 .00002 Thermal neutron scatter = 10 barns
Neptunium 236 154000 2800 7
237 2144000 .019 60
238 .0058 Beta
239 175.9 .0065 Beta
Plutonium 237
238 558 87.7 Alpha 16.8 9.5
239 1017.3 14100 Alpha 748 10 Thermal neutron scatter = 8 barns
240 289.6 6561 Alpha .030 40
241 363 14.3 Beta 937 12
242 18.5 373000 Alpha 80
243 87.4 .00057 Beta
244 1.7 80.8 Alpha
Americium 241 748 432 3.1 60
243 75.3 7370 .0044 200
244 .00127 Beta
Curium 242 20
243 29.1 690 8
244 16.2 18.1 Alpha 15
245 383 8500 Alpha 2161 10
246 1.36 4730 Alpha 45
247 58 15700000 Alpha 7
248 2.49 340000 Alpha
249 .000122 Beta
250 9000
251 .000032 Beta
Berkelium 247 710 1379 Alpha 76
249 1600 .90 Beta 192
250 .00037 Beta
251 .000106 Beta
Californ 249 481.4 351 600 6
250 1701 13.08 Alpha
251 2849 900 Alpha 4801 5.46
252 20.4 2.64 Alpha 2.73
253 12 .049 Beta
254 .166 SF
Einstein 253 .056 Alpha
254 .75 9.89
Cross section Melt
barns Kelvin
Oxygen .00019
Carbon .0035 3800 Graphite
Beryllium .0092 1560
Bismuth .034 545
Magnesium .063 923
Lead .171
Silicon .171 1687
Zirconium .184 2128
Aluminum .232 933
Hydrogen .333
Tin .626 505
Zinc 1.11 693
Niobium 1.15
Iron 2.56 1811
Molybdenum 2.6
Chromium 3.1
Copper 3.78 1358
Nickel 4.49
Titanium 6.09
Thorium 7.37
Uranium 7.57 1405
Tungsten 18.3
Tantalum 20.6
Xenon 23.9
Krypton 25
Chlorine 35.5
Hafnium 104 2506
Indium 194
Mercury 374
Iridium 425
Boron 767 2349
Dysprosium 920
Plutonium 1017 912
Cadmium 2450 594
Europium 4600
Samarium 5922
Gadolin 49000
Blue elements are unstable with a half life much less than the age of the solar system.
The only elements heavier than Bismuth that can be found on the Earth are Thorium and Uranium, and these are the only elements that can be tapped for fission energy.
Natural Thorium is 100% Thorium-232
Natural Uranium is .72% Uranium-235 and 99.3% Uranium-238.
Plutonium doesn't exist in nature.
Protons Neutrons Halflife Critical Isotope
(106 yr) mass (kg) fraction
Thorium-232 90 142 14000 - 1.00 Absorbs neutron -> U-233
Uranium-233 92 141 .160 16 - Fission chain reaction
Uranium-235 92 143 700 52 .0072 Fission chain reaction
Uranium-238 92 146 4500 - .9927 Absorbs neutron -> Pu-239
Plutonium-238 94 144 .000088 - - Produces power from radioactive heat
Plutonium-239 94 145 .020 10 - Fission chain reaction
The elements that can be used for fission energy are the ones with a critical
mass: Uranium-233, Uranium-235, and Plutonium-239.
Uranium-233 and Plutonium-239 can be created in a breeder reactor.
Thorium-232 + Neutron -> Uranium-233 Uranium-238 + Neutron -> Plutonium-239The "Fission" simulation at phet.colorado.edu illustrates the concept of a chain reaction.
Natural uranium is composed of .7% Uranium-235 and the rest is Uranium-238. Uranium-235 can be separated from U-238 using centrifuges, calutrons, or gas diffusion chambers. Uranium-235 is easy to detonate. A cannon and gunpowder gets it done.
Plutonium-239 is difficult to detonate, requiring a perfect spherical implosion. This technology is beyond the reach of most rogue states.
Uranium-233 cannot be used for a bomb and is hence not a proliferation risk.
Plutonium-238 emits alpha particles, which can power a radioisotope thermoelectric generator (RTG). RTGs based on Plutonium-238 generate 540 Watts/kg and are used to power spacecraft.
The fission of uranium-233, uranium-235, and plutonium-239 yields similar energies. The "reactor heat" column is the energy yield per nucleus in a reactor. Energies in MeV:
Fission Prompt Prompt Prompt Decay Decay Anti- Reactor
fragments neutrons gammas neutron betas gammas neutrinos heat
capture
Uranium-233 168.2 4.9 7.7 9.1 5.2 5.0 6.9 200.1
Uranium-235 169.1 4.8 7.0 8.8 6.5 6.3 8.8 202.5
Plutonium-239 175.8 5.9 7.8 11.5 5.3 5.2 7.1 211.5
Creating Plutonium-239 and Uranium-233:
Uranium-238 + Neutron -> Plutonium-239 Thorium-232 + Neutron -> Uranium-233 Detail: Uranium-238 + Neutron -> Uranium-239 Uranium-239 -> Neptunium-239 + Electron + Antineutrino Halflife = 23 mins Neptunium-239 -> Plutonium-239 + Electron + Antineutrino Halflife = 2.4 days Thorium-232 + Neutron -> Thorium-233 Thorium-233 -> Protactinium-233 + Electron + Antineutrino Halflife = 22 mins Protactinium-233 -> Uranium-233 + Electron + Antineutrino Halflife =
When a nucleus absorbs a neutron it can either fission or it can capture the neutron and transmute to another element. If it captures the neutron then it doesn't generate fission energy and it becomes "actinide waste". The higher the fission-to-capture ratio the better.
Fission to Outcome of
capture ratio neutron capture
Uranium-233 10 Uranium-235
Uranium-235 6 Plutonium-239
Plutonium-239 2 Plutonium-240 Halflife = 6500 years
Plutonium-241 4 Plutonium-242 Halflife = 373000 years
Uranium-233 + Neutron -> Uranium-234 Halflife = 246000 years
Uranium-234 + Neutron -> Uranium-235 Uranium-234 neutron cross section = 100 barns
The thorium fuel cycle generates less transuranic waste than the uranium fuel cycle.
If thorium is used to breed Uranium-233 then the Uranium-233 either fissions
or becomes Uranium-235, when then fissions. Hence almost all of the original
thorium ends up fissioning.
Uranium-235 Uranium-233 Price
($/kg)
Krypton-83 .00536 .0101 330
Molybednum-95 .0654 .0636 24
Ruthenium-101 .0517 .0317 5600
Rhodium-103 .0304 .0157 88000
Silver-109 .000322 .000395 590
Cadmium-113 .000143 .000135 1.9
Indium-115 .000124 .000144 750
Tin-125 .000347 .00117 22
Iodine-127 .00160 .005563 16
Xenon-131 .0290 .0360 1200
Xenon-134 .0784 .0630 1200
Xenon-136 .0609 .0667 1200
Barium-134 7.7e-8 .0000027 100
Barium-137 0 0 100 Slowly generated by Caesium-137
Neodymium-143 .0596 .0597 25
Neodymium-145 .0394 .0345 25
Gadolinium-154 1.1e-13 6.2e-12 20
Gadolinium-155 2.9e-11 1.5e-10 20
Gadolinium-156 .000150 .000128 20
Gadolinium-157 .0000624 .0000631 20
Gadolinium-158 .0000335 .0000216 20
The elements that are valuable enough to be worth extracting are:
Uranium-235 Uranium-233 Price
($/kg)
Krypton .00536 .0101 330
Ruthenium .0517 .0317 5600
Rhodium .0304 .0157 88000
Silver .000322 .000395 590
Indium .000124 .000144 750
Xenon .1683 .1657 1200
Ruthenium, Rhodium, and Xenon are the best candidates for extraction. Xenon is
easy to extract because it's a gas. Xenon is a miraculous highly-safe anaesthetic.
Uranium costs 75 $/kg. If 1 kg of spent Uranium fuel contains 3% fission products then it contains .91 grams of rhodium, which is worth 80 $.
Strontium-90 is a radioactive product of fission that is useful for nuclear batteries.
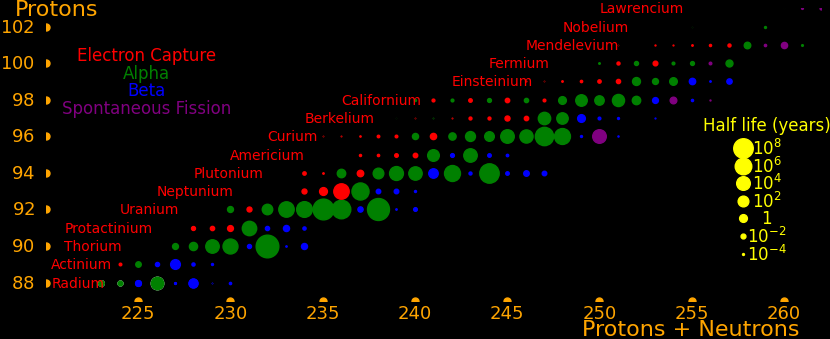 |
|---|
The actinides are the elements from actinium to lawrencium. None are stable but many are long-lived.
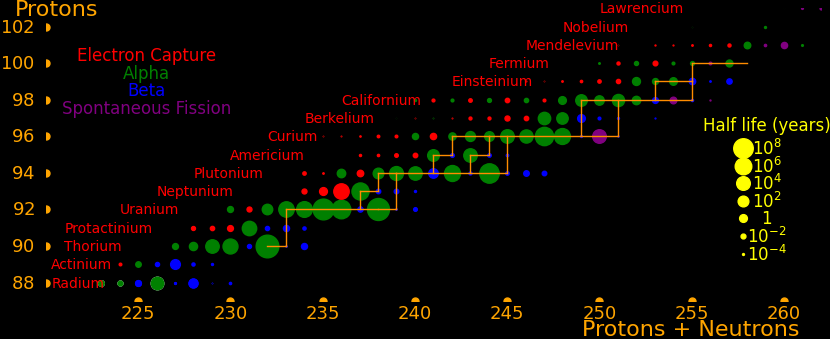 |
|---|
Neutron capture transmutes an isotope one space to the right and beta decay transmutes an isotope one space up.
The most massive nuclei that exist naturally are thorium-232, uranium-235, or uranium-238. All are unstable but have half lives larger than 700 million years. The road starts with these isotopes and then adding neutrons transmutes them according to the orange lines. The road forks at beta isotopes, which can either beta decay or capture a neutron.
The end of the road is fermium. Neutrons can't further increase the proton number because no fermium isotopes on the road beta decay. The road goes as far as fermium-258, which has a half life of .00037 seconds and spontaneously fissions. Producing heavier isotopes requires an accelerator or an extreme neutron flux (such as occurs in a fission bomb).
Most of the long-lived isotopes are on the neutron road, the most significant exceptions being neptunium-236 and berkelium-247. These isotopes can be reached by alpha decay, which moves an isotope 2 spaces down and 4 to the left.
Americium-242m (half live 141 years) is an excited state of Americium-242 (half life .0018 years) with a high thermal neutron capture cross section.
The thermal neutron capture cross section of Americium-241 to Americium-242 is 748 barns, and to Americium-242m is 83.8 barns.
 |
|---|
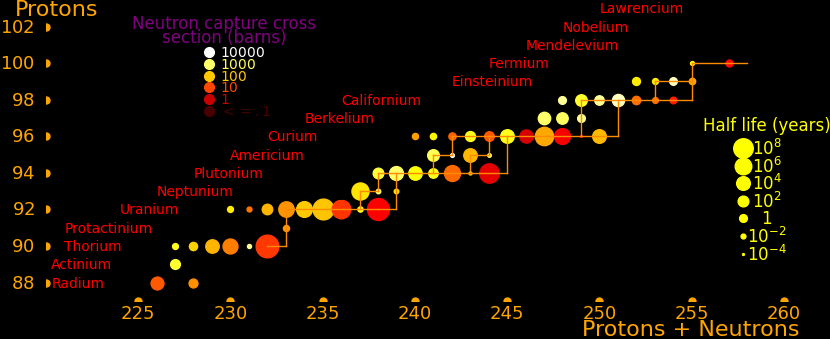
Transmutation rate is proportional to the neutron capture cross section. In order to move rightward on the road the isotope has to have a large neutron capture cross section and it has to have a large half life. This is true everywhere on the road except for curium-249, and so all the long-lived isotopes on the road are easily created, except for curium-250.
The road has a bottleneck at curium-246, which is the isotope with the lowest capture cross section (1.36 barns). The capture cross section of curium-248 is also low (2.49 barns). Traffic slows down here and all the isotopes further down the road have to wait for curium-246 and curium-248.
To create curium-250 you start with curium-248 and add a neutron to produce curium-249. Curium-249 has a half life of 64 minutes and you have to hope it captures a neutron before the decay.
 |
|---|
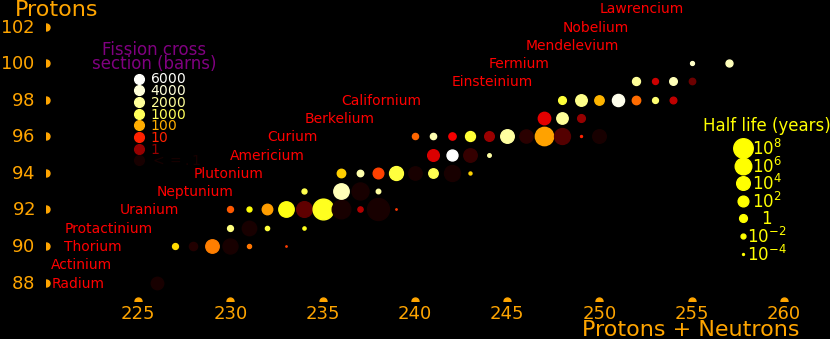
The fission cross section is for thermal neutrons with a Maxwellian spectrum centered at .025 eV. The isotopes with large fission cross sections are:
Thermal Critical Half life
neutron mass
fission
barns kg years
Americium-242m 6686 11 141
Californium-251 4801 5.5 900
Einsteinium-254 2900 9.9 .75
Neptunium-236 2800 6.8 154000
Curium-245 2161 10 8500
Californium-249 1665 6 351
Plutonium-241 937 12 14.3
Plutonium-239 748 10 24100
Curium-243 690 8 29.1
Uranium-235 538 52 704000000
Uranium-233 468 15 159200
 |
|---|
 |
|---|
 |
|---|
Fast Crit Crit Half life Fast Fast
neutron mass diam neutrons capture
fission /fission
barns kg cm years barns
Californium-252 2.32 2.73 4.30
Californium-251 1.28 5.46 900 4.56 .63r
Californium-249 1.74 6 351 *
Curium-247 1.86 7.0 *
Neptunium-236 7 154000 *
Curium-243 2.43 8 29.1 3.70 .4
Plutonium-238 1.994 9.5 3.148
Einsteinium-254 9.89 .75 *
Curium-245 1.75 10 8500 4.0 .4
Plutonium-239 1.800 10 24100 3.123
Americium-242m 1.83 10 141 3.53 .6
Plutonium-241 1.648 12 14.3 3.142
Curium-244 1.73 15 3.52 .8
Uranium-233 1.946 15 159200 2.649
Uranium-235 1.235 52 704000000 2.606
Plutonium-240 1.357 40 3.061
Curium-246 1.25 45 3.49 .4
Neptunium-237 1.335 60 2.889 1.8
Berkelium-247 75.7
Plutonium-242 1.127 80 3.07
Americium-241 1.378 60 3.457 2.0
Berkelium-249 192 3.74
Americium-243 .2i 200 3.45 1.8
Einsteinium-254m
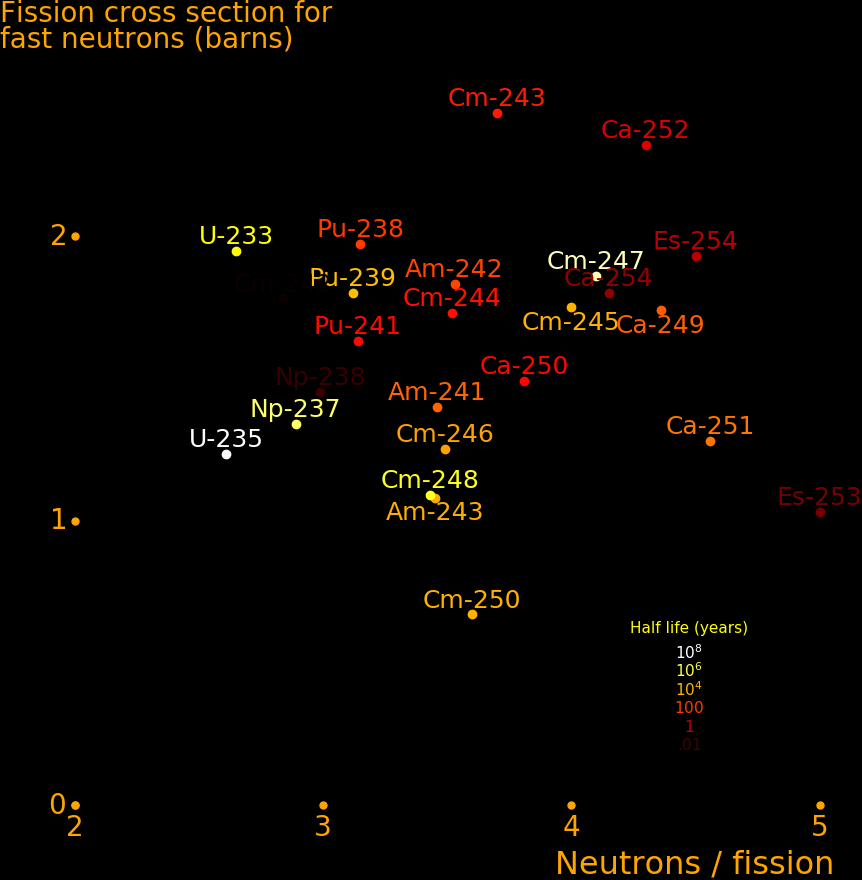
Isotopes with a neutron capture cross section of 1 barn or more can be transmuted on a timescale of 10 years. Isotopes with a cross section smaller than this can't be practically transmuted.
To calculate the transmutation rate,
Neutron flux = F = 10-8 neutrons/barn/second Neutron capture = A = 10 barns Transmutation rate = R = FA = 10-7 transmutations/second = 3.2 transmutations/year
 |
 |
|---|---|
If a nucleus is hit with a pulse of neutrons then the probability that a fission occurs is:
Thermal neutron fission cross section = A = 6400 barns = 6.4⋅10-25 meters2 For Americium-242m Neutron pulse magnitude = F = 1020 neutrons/meter2 Fission probability = P = AF = 6.4⋅10-5 fissions
Actinides are useful for:
*) Neutron-induced fission
*) Radioactivity heat
*) Spontaneous fission
All of these properties are useful for spacecraft. The most useful actinides are:
Half life Neutron Spontaneous Radioactivity
fission fission
years barns Watts/kg Watts/kg
Uranium 233 159200 468
235 704000000 538
Plutonium 238 87.7 818
239 14100 748
241 14.3 937 4315
Americium 241 432
242m 141 6686
Curium 243 29.1 690 2666
244 18.1 4014
245 8500 2161
246 4730
247 15700000
248 340000 .64 .81
250 9000 240 241
Berkelium 249 .90
Californium 248 .91 86 86209 Off-road
249 351 1665
250 13.08 158 5778
251 900 4801
252 2.64 31227 58470
253 .049
254 .166 15896000 15897000
Einsteinium 254 .75 2900
Fermium 257 .275 ? 20000 279000
After Before
ppt ppt
U-234 .2
U-235 10.3 33
U-236 4.4
U-238 943 967
Pu-238 .18
Pu-239 5.7
Pu-240 2.21
Pu-241 1.19
Pu-242 .49
Np-237 .43
Am-241 .22
Am-242 .0007
Am-243 .10
Cm-242 .00013
Cm-243 .00032
Cm-244 .024
Fission products 35
Tc-99 .81
Neutron flux (Neutrons/cm2/second)
Power reactor 5e13
High-flux reactor 6e15
Cosmological s-process e16
Cosmological r-process e27
Fission bomb e31
Thermal Fast Crit Crit Half life Slow Fast SF Therm Fast Fast SF SF
neutron neutron mass diam neutr neut neut capt capt inel
fission fission /fiss /fiss /fiss barn barn W/kg neut/s/kg
barns barns kg cm years
Thorium-232 .078 2.16
Protactinium-231 .83 2.457
Uranium-232 80 2.013 3.296 2
Uranium-233 468 1.946 15 159200 2.48 2.649 73
Uranium-234 .407 1.223 2.578 1.8 3.9
Uranium-235 538 1.235 52 704000000 2.42 2.606 2.0 690 .0057
Uranium-236 .042 .594 2.526 1.8 2.3
Uranium-238 .00001 .308 2.601 1.97 2.68 5.51
Neptunium-236 2800 * 7 154000 * *
Neptunium-237 .019 1.335 60 2.54i 2.889 2 1.8 <.05
Neptunium-238 1243 1.45 .0058 2.79i 2.99i .1
Plutonium-237 2100i .124 * *
Plutonium-238 16.8 1.994 9.5 87.7 2.36 3.148 2.28 558 1204000 Alpha
Plutonium-239 748 1.800 10 24100 2.87 3.123 2.9 1017.3 10.1 Alpha
Plutonium-240 .030 1.357 40 6560 3.061 2.189 478000
Plutonium-241 937 1.648 12 14.3 2.92 3.142 36 <.8 Beta
Plutonium-242 .0026 1.127 80 373000 3.07 2.28 805000
Plutonium-243 181i
Plutonium-244 80800000
Plutonium-245 8500
Americium-241 3.1 1.378 60 3.12 3.457 2.0 500
Americium-242 1322i 3.4i .7
Americium-242m 6686 1.83 10 141 3.26 3.53i 2 .6
Americium-243 .2 .2i 200 3.20i 3.45i 1.8
Americium-244 1528i 3.4i * * .9
Americium-244m 1220i 3.4i 3.14i 3.42i .8
Curium-241 2600 2.21 .090
Curium-242 5 1.78 2.54
Curium-243 690 2.43 8 29.1 3.43 3.70i .4
Curium-244 1.1 1.73 15 18.1 2.72? 3.52i 16.2 .8 3.24i(t) Alpha
Curium-245 2161 1.75 10 8500 3.83 4.0 383 .4 Alpha
Curium-246 .17 1.25 45 2.93 3.49i .4 3.19i(t)
Curium-247 82 1.86 7.0 15700000 3.80 * 58 Alpha
Curium-248 .34 1.09 3.13 * .64
Curium-249 1.21
Curium-250 * .67 3.30 *
Berkelium-247 * 75.7
Berkelium-249 1.0 * 192 3.40 3.74i 240
Berkelium-250 959i
Californium-246 3.1
Californium-248 1.32 86
Californium-249 1665 1.74 6 351 4.06 * 3.4 481.4
Californium-250 112 1.49 3.51 * 158
Californium-251 4801 1.28 5.46 900 4.1 4.56 2839 .62 2.216 Alpha
Californium-252 33 2.32 2.73 2.64 4.00i 4.30i 3.75 20.4 31227 Alpha
Californium-253 1138 * * *
Californium-254 2.001j 1.80 .75 3.85 *
Einsteinium-253 2.51 * 4.7 * 15.9M
Einsteinium-254 2900 * 9.89 .75 4.2 *
Einsteinium-254m 1840 * * *
Fermium-244 4
Fermium-246 4
Fermium-254
Fermium-255 3360i * 4 *
Fermium-256 3.63 *
Fermium-257 * * .275 3.87 * 20000
Nobelium-252 4.2
Thermal Fast Critical Diam Half life Slow Fast SF SF Spontaneous
neutron neutron mass neutrons neutrons neutrons fission
fission fission /fission /fission /fission W/kg neutron/s/kg
barns barns kg years
The prompt kinetic energy released by fission is:
Fission energy (MeV)
Actinium 168
Thorium 172
Protactinium 177
Uranium 181
Neptunium 185
Plutonium 189
Americium 195
Curium 198
Berkelium 203
Californium 207
 |
|---|
Natural Thermal Half life Decay Thermal Warm Fast 14 MeV Fast Mass
fraction neutron mode neutron neutron neutron capture
capture scatter scatter scatter
barns years barns barns barns barns barns AMU
Neutron 1 0 611s Beta 1.00866491588
Hydrogen 1 .99985 .3326 - 32.8 20 3.93 .69 .000039 1.00782504
2 .00015 .000519 - 4.70 6 2.53 .0062 .0000071
3 0 0 12.32 Beta
Helium 3 1.34m 5333 - 4.10 5 2.12 .95 .82 3.01602932265
4 1 0 - .96 .9 3.69 1.05 0 4.00260325413
5 0 7e-22s Neutron 5.012057
6 0 .806s Beta 6.01888589
Lithium 6 .075 941 - .90 1.42 .91 6.0151228874
7 .925 .0454 - 7.016003437
8 0 .839s Beta 8.02248625
Beryllium 6 0 5e-21s 2p 6.019726
7 0 .145 EC 7.01692872
8 0 8e-17s Alpha 8.00530510
9 1 .0085 - 7.33 8 2.68 .98 .0000013 9.01218307
10 0 1390000 Beta 10.01353470
11 0 13.8s Beta 11.02166108
12 0 .022s Beta 12.0269221
Boron 9 0 8e-19s P,Alpha
10 .199 200 - 2 2 2 .4
11 .801 -
12 .020s Beta
Carbon 12 .002 5 7 2 .00001
Oxygen 16 .0001 4 6 3 3e-8
Fluorine
 |
 |
|---|---|
 |
 |
|---|---|
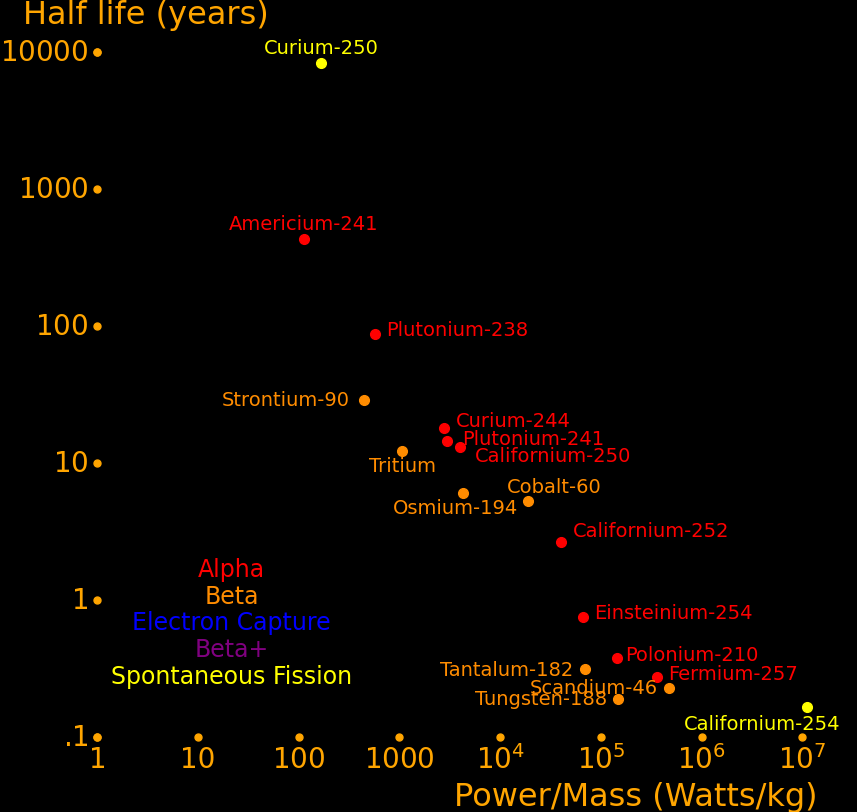
The most important radioactive isotopes for power are:
Strontium-90 Abundant because it is present in burnt fission fuel.
Caesium-137 Abundant because it is present in burnt fission fuel.
Plutonium-238 Outperforms Strontium-90. Has to be bred in a reactor.
Capable of powering a helicopter for 50 years.
Cobalt-60 Larger power/mass than plutonium-238.
Capable of powering a helicopter with more acceleration than a human can handle.
Californium-252 Superlatively large power/mass. Capable of powering an Iron Man suit.
Beryllium-7 Largest power-mass of all isotopes with a half life larger than .1 years.
Curium-250 Only isotope that decays primarily by spontaneous fission and hence
has a vastly higher energy/mass than the other isotopes.
Halfnium-172 Large power/mass. Of interest because HfC has the highest melting point of all known materials.
Plutonium-241 Easy isotope to produce. Good balance of power/mass and half life.
Curium-244 Good balance of power/mass and half life.
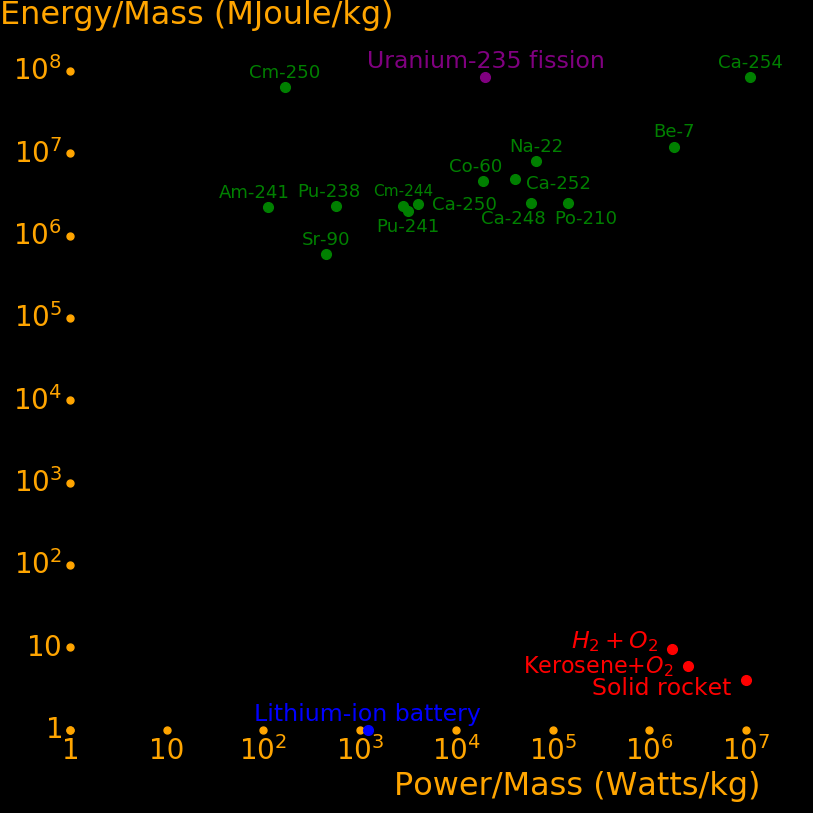
The most important radioactive isotopes are:
Power/Mass Energy/Mass Halflife Decay Decay
Watts/kg GJoules/kg years MeV mode
Californ-254 15900000 83538 .166 220 SF
Beryllium-7 2577000 11875 .146 .8619 EC
Scandium-46 686400 4961 .229 2.366 β
Polonium-210 207730 2485 .379 5.41 α
Curium-242 176110 2479 .446 6.22 α
Einsteinium-254 105432 2512 .755 6.616 α
Sodium-22 97237 7979 2.6 1.82 β+ or EC
Manganese-54 91144 2459 .855 1.377 EC
Californium-248 86210 2476 .91 6.36 α or SF
Californium-252 58470 4871 2.64 6.12 α or SF
Cobalt-60 27300 4533 5.27 2.82 β,γ
Californium-250 5779 2385 13.1 6.02 α or SF
Plutonium-241 4315 1961 14.4 4.90 α
Curium-244 4014 2293 18.1 5.80 α
Krypton-85 3962 1350 10.8 1.19 β,γ
Halfnium-172 3210 189 1.87 .3378 EC
Curium-243 2666 2449 29.1 6.169 α
Caesium-137 1736 1654 30.2 2.35 β
Hydrogen-3 1538 598 12.32 .0186 β
Plutonium-238 818 2265 87.7 5.59 α
Strontium-90 648 589 28.8 .55 β
Curium-250 241 63170 8300 5.17 SF or α
Americium-241 161 2197 432 5.49 α
Radium-226 42.0 2121 1600 4.97 α,γ
"Neutrons" is the number of neutrons required to produce the isotope in a fission reactor.
Power/Mass Energy/Mass Halflife Decay Decay Neutrons Production
Watts/kg GJoules/kg years MeV mode required method
Neptunium-239 .00645 β
Fermium-253 .0082
Calif-253 .0488
Calif-254 .166
Fermium-257 .275
Neptunium-235 1.084
Calif-249 351
Calif-251 898
Berkelium-247 1380
Thorium-229 7340
Curium-245 8500
Plutonium-239 24110
Lithium-8 .839s β
Beryllium-11 13.76s
Beryllium-7 2577000 11875 .146 .8619 EC Deuteron
Cobalt-56 912300 6104 .212 3.544 β+ Accelerator
Scandium-46 686400 4961 .229 2.366 β Neutron
Polonium-210 207730 2485 .379 5.41 α 1 Neutron
Curium-242 176110 2479 .446 6.22 α - Neutron
Einsteinium-254 105432 2512 .755 6.616 α Neutron
Sodium-22 97237 7979 2.6 1.82 β+ or EC - Deuteron
Manganese-54 91144 2459 .855 1.377 EC Deuteron
Californium-248 86210 2476 .91 6.36 α or SF Accelerator
Rhodium-102 67003 1199 .567 1.268 β+ or β Accelerator
Cobalt-57 60237 1414 .744 .8359 EC Deuteron
Californium-252 58470 4871 2.64 6.12 α or SF 14 Neutron
Einsteinium-252 51348 2090 1.29 5.46 α or EC Accelerator
Thulium-170 49436 549 .352 .968 β Neutron
Vanadium-49 41572 1185 .903 .6019 EC Deuteron
Thorium-228 38697 2335 1.912 5.520 α Accelerator
Iridium-192 29942 191 .202 .38 β Neutron
Cobalt-60 27300 4533 5.27 2.82 β,γ Neutron
Plutonium-236 26583 2398 2.858 5.867 α Accelerator
Polonium-208 26446 2420 2.898 5.22 α - Accelerator
Zinc-65 23254 490 .667 .3299 β+ Neutron
Caesium-134 22795 1482 2.06 2.059 β
Tungsten-181 9546 100.0 .332 .1877 EC Accelerator
Cerium-144 8679 214 .780 .319
Lutetium-173 8645 374 1.37 .6705 EC
Californium-250 5779 2385 13.1 6.02 α or SF 12 Neutron
Promethium-146 5659 988 5.53 1.495 EC or β Accelerator
Rhodium-101 4967 517 3.3 .5417 EC Deuteron
Iron-55 4706 405 2.73 .2312 EC,γ Neutron
Plutonium-241 4315 1961 14.4 4.90 α Neutron
Curium-244 4014 2293 18.1 5.80 α 6 Neutron
Krypton-85 3962 1350 10.8 1.19 β,γ Neutron
Halfnium-172 3210 189 1.87 .3378 EC Accelerator
Curium-243 2666 2449 29.1 6.169 α Neutron
Lutetium-174 1868 195 3.31 .3521 β+
Promethium-147 1777 147 2.62 .224 β 1 Neutron
Caesium-137 1736 1654 30.2 2.35 β 0 Fission product
Hydrogen-3 1538 598 12.32 .0186 β 1 Lithium-6 + Neutron -> Alpha + Tritium
Ruthenium-106 1116 35.8 1.018 .0394 β
Europium-152 1194 509 13.5 .802 EC,β-+,γ 1
Europium-155 1048 157 4.753 .2527 β
Uranium-232 1035 2251 68.9 5.414 α Accelerator
Tantalum-179 991 56.9 1.82 .1056 EC
Gadolinium-148 876 2072 75 3.18 α Accelerator
Plutonium-238 818 2265 87.7 5.59 α 3 Neutron
Europium-150 684 796 36.89 1.238 β+
Strontium-90 648 589 28.8 .55 β 0 Fission product
Polonium-209 582 2298 125.2 4.98 α - Accelerator
Titanium-44 295 587 63 .2676 EC
Curium-250 241 63170 8300 170 SF or α Neutron High neutron flux required
Promethium-145 194 108 17.7 .163 EC -
Americium-241 161 2197 432 5.49 α -
Silicon-32 140 675 153 .224 β
Radium-228 107 19.4 5.75 .0458 β -
Radium-226 42.0 2121 1600 4.97 α,γ -
Curium-246 14.4 2170 4760 5.48 Neutron
Plutonium-240 10.1 2094 6564 5.21 α,γ 2
Americium-243 9.28 2159 7370 5.439 α
Carbon-14 5.94 1075 5730 .156 β 2
Chromium-51 .0758
Thallium-204 3.773 .7638 β
Barium-133 10.52 .5175 EC
Antimony-125 2.759 .7667 β
Gold-195 .460 .2268 EC
Niobium-93m 16.13
Calcium-45 .445
Bismuth-207 31.6 1.375 β+
Cadmium-109 1.267 .2142 EC
Neptunium-235 1.084 .1242 EC
Thulium-171 1.92 .0965 β
Lead-210 22.2 .0635 β
Ruthenium-106 1.02 .039 Beta
Nickel-63 100.1 .0670 Beta
Osmium-194 6.0 .0966 β
 |
|---|
A fission afterburner uses fission fuel as exhaust, with fission triggered by neutrons from a reactor.
The reactor operates in pulse mode. The reactor produces a pulse of neutrons that trigger fission in the fuel, and then the fuel is expelled. The reactor then has to cool down before generating another pulse.
A TRIGA-style reactor can produce millisecond neutron pulses. The pulse is initiated by neutrons from spallation, where high-energy protons from an accelerator strike a tungsten target and eject neutrons from tungsten nuclei. Fuel can be confined magnetically for the duration of the pulse.
Fission fuel should have a large fission cross section for thermal neutrons, and the best isotopes are:
Half life Fission Energy Quality Exhaust Neutron capture
year barn MeV meter/s output
Americium-242m 141 7024 195 5640 93 Daughter nuclei + Neutrons
Californium-251 900 4801 207 3940 78 Daughter nuclei + Neutrons
Curium-245 8500 2161 198 1740 52 Daughter nuclei + Neutrons
Plutonium-239 14100 748 189 590 30 Daughter nuclei + Neutrons
Uranium-235 704000000 538 181 410 25 Daughter nuclei + Neutrons
Beryllium-7 .146 56800 1.644 11670 134 Lithium-7 + Proton
Helium-3 Stable 5333 .764 1020 40 Tritium + Proton
Boron-10 Stable 3835 2.34 820 35 Lithium-7 + Alpha
Lithium-6 Stable 940 4.783 640 31 Alpha + Tritium
Fuel quality is given by:
Neutron capture cross section = A meter2 At 300 Kelvin Fission energy = E Joule Mass = M kg Mass of target + mass of neutron Fuel quality = Q = AE/M
Neutrons are chilled to liquid helium temperature before encountering the fuel, to increase the neutron capture cross section. Cross sections in the table are for 300 Kelvin.
The exhaust speed is:
Room temperature = T = 300 Kelvin Helium boiling point = t = 4.2 Kelvin Neutron chill factor = C = (T/t)½ = 8 Dimensionless Neutron capture cross section at 300 Kelvin= A meter2 Neutron capture cross section at 4 Kelvin = CA meter2 Neutron number density = n = 1019 neutrons/meter2 Target number density = N = 1/A Nuclei/meter2 Number density of fuel nuclei Fraction of targets that capture neutrons = F = n/N Exhaust energy/mass = e = CFE/M = CnAE/M Exhaust speed = V = (2e)½
The fuel number density should be large enough to capture most of the neutrons, and not larger, and this corresponds to "tA=1". At this density, most neutrons are captured. Only a small fraction of targets get neutrons. There are never enough neutrons to fission all the fuel, hence the goal is to maximize neutron density.
The fission reactor produces a neutron pulse with a density of order 1019 neutrons/meter2 and timescale of order 1 millisecond. During the pulse, the uranium in the reactor heats up by of order 3500 Kelvin. We assume 1 ton of uranium. The reactor pulse is initiated by a pulse of neutrons from spallation.
Uranium melting point = 1405 Kelvin Uranium boiling point = 4404 Kelvin Uranium melt energy = 38900 Joule/kg Uranium heat capacity = 118 Joule/kg Uranium temperature change = 3500 Kelvin Uranium heat energy change per mass = .413 MJoule/kg Uranium heat per neutron = 200 MeV/neutron Neutrons per kg of uranium = 2.6e16 Neutrons/kg Uranium mass = 1000 kg Neutrons in the pulse = 2.6e19 Neutrons Uranium density = D = 17300 kg/meter3 (liquid) Uranium radius = .24 meter Inner sphere Reactor radius = .4 meter Includes an outer shell of moderator Neutron density = 1.3e19 Neutrons/meter3
The rocket consists of concentric spherical shells, with shell 1 the innermost.
Shell 1: Contains the nuclear reactor that generates neutrons.
Shell 2: Beryllium oxide moderator to slow neutrons to room temperature.
Shell 3: Liquid helium moderator to further slow the neutrons
Shell 4: Pressure vessel containing the fission fuel and exhaust gas
Shell 5: Liquid helium moderator to return neutrons back to shell 3.
The more compact the reactor and moderator, the better. The moderator with the largest hydrogen density is TaD5.
The stopping length of a neutron in Americium-242m is:
Cross section = A = 6686 barns Atomic mass unit = u = 1.660e-27 kg Nucleons = q = 242 Nucleus mass = M = 4.02e-25 kg Atom density = N = D/u = 2.99e28 atoms/meter3 Density = D = N u q = 12000 kg/meter3 Neutron stopping length = X = 1/(AN) = 5.00e-5 meters Americium-242m mass/Area= m = D X = .6 kg/meter2
A steel gun can achieve a chamber pressure of 4⋅108 Pascals and a tungsten gun can achieve a chamber pressure of 109 Pascals.
Neutrons trigger fission in lithium-6 and the fission fragments can act as exhaust.
Lithium-6 + Neutron -> Alpha + Tritium + 4.78 MeV
For an ion drive powered by a nuclear reactor, neutrons are being produced for free and they can be harnessed for thrust with lithium-6. The aft side of the spacecraft is coated with a thin layer of lithium-6 and the neutrons trigger fission.
Lithium-6 is the best isotope for triggered fission propulsion because it has a high neutron fission cross section and because a large amount of energy is released per fission.
A neutron trigger rocket uses thermal neutrons to trigger the release of energy in a target isotope. Some isotopes, upon capturing a thermal neutron, decay immediately and produce energy. The most significant isotopes are:
1 1/64 1/16 .00098 1/200 1/4 1/4 .00031
Source Trigger Cross section Energy Energy/Mass Fragment Escape Fragment Decay output
prowess barns MeV MeV/nucleon momentum number energy
MeV⋅barn/nucleon efficiency efficiency
Uranium-235 538 1.9 .00424 2.52 .0203 Fission fragments + Neutrons
Californium-251 4801 Fission fragments + Neutrons
Plutonium-239 748 2.0 .00417 2.95 Fission fragments + Neutrons
Gadolinium-157 12780 254000 7.9 - 1 Gamma
Americium-242m 5818 6400 220 .241 Fission fragments + Neutrons
Helium-3 1350 5320 .764 .255 .188 2 .287 Proton + Tritium
Lithium-6 749 940 4.78 .797 .245 2 2.34 Alpha + Tritium
Boron-10 883 3837 2.3 .230 .231 1 .531 Alpha + Lithium-7
Polonium-210 5.41 .0196 1 .106 Alpha + Lead-206
Boron-10 347 303 11.45 - 1 Gamma
Lithium-7 .0454 2.03 - 1 Gamma .84 seconds
Lithium-6 47 39 7.25 - 1 Gamma
Hydrogen-1 .7 .333 2.23 2.23 - 1 Gamma
Plutonium-239 748 211.5 .885 - Fission fragments + Neutrons
Lithium-8 16.10 Beta
Beryllium-8 .0918 Alpha
The "trigger prowess" reflects the energy/mass extracted from the target.
Fragmentation energy = H Fragment momentum efficiency = Q Escape number = g Fragment energy efficiency = k = HQg Heat efficiency = q Exhaust energy efficiency = K = HQgq Neutron capture cross section = A Energy yield = E Number of nucleons = m Trigger prowess = P = AE/m
Gamma and beta decays are not useful for rockets because these particles interact weakly with matter, plus the recoil nucleus has low momentum and energy.
A "heavy decay" is a decay that contains nucleons, such as an alpha, proton, or fission fragment. A heavy decay is useful for rockets because these particles interact strongly with matter and all of their energy goes into the exhaust gas.
The most useful isotopes for a rocket are Americium-242m, Helium-3, Lithium-6, and Boron-10.
Helium-3 and Lithium-6 can be used directly as exhaust because all the decay particles are lightweight.
Momentum efficiency = f = .245 Escape efficiency = .25 Energy ratio = .0226 Total efficiency = .00138 Reactor energy/mass = 2000 Watts/kg Exhaust energy/mass = 2.8 Watts/kg Disintegration energy = 4.78 MeV Fission energy =211.5 MeV Tritium stopping length =.000394 meter Alpha stopping length =.000074 meter Neutron stopping length =.000092 meter Total efficiency
Cross section = A = 940 barns Lithium-6 density = D = 530 kg/meter2 Atomic mass unit = m = 1.660e-27 kg Lithium-6 mass = M = 6 m = 9.96e-27 Lithium-6 number/volume = N = D/M = 5.32e28 atoms/meter3 Lithium-6 thickness = X = .0004 meters Lithium-6 number/area = n = XN = 2.13e25 atoms/meter2 Hits/area = h = 1.38e21 hits/meter2 Stopping length = L = 1/(AN) = .000200 meters Lithium-6 decay energy = E = 4.78 MeV Exhaust speed = V = 100 km/s Lithium-6 energy/mass = e = 76900 GJoules/kg Exhaust energy/mass = e = 5 GJoules/kg Hit fraction = f =.000065 Plutonium-239 energy = 211.5 MeV
Fission heat = H = 211.5 MeV Neutrons/fission = N = 2 Decay energy = E = 4.78 MeV Rocket efficiency = Z = NE/H = .045 Reactor power/mass= p = 800 Watts/kg Exhaust power/mass= p = 36 Watts/kg Energy = E Mass = M Energy/mass = e Momentum = Q Momentum/mass= q = V Acceleration = A
Fission cross section = A Fuel density = D Nucleus mass = m Nucleus number density = n = D/m Fuel heat capacity = C = 116 Joules/kg/Kelvin Fuel max temperature = T Fuel temperature change = t
The neutron density should be as large as possible, which is achieved by pulsing the reactor. A pulse produces a swarm of neutrons, raises the temperature of the reactor, and then the reactor has to cool back down for another pulse. To estimate the number of neutrons produced by a reactor pulse,
Reactor temperature increase = T = 1000 Kelvin Plutonium heat capacity = C = 115 Joules/kg/Kelvin Change in Energy/Mass = e = CT = 115000 Joules/kg Fission fuel mass = M Reactor heat energy gain = H = MCT Heat per fission = h = 189.5 MeV Number of fissions = F = H/h Thermal neutrons per fission = n = 1 Varies according to reactor design Thermal neutrons produced = N = Fn
To calculate the neutron density,
Plutonium density = D =19800 kg/meter3 Plutonium volume fraction = f = .1 Volume fraction of plutonium in the reactor Reactor size = L Plutonium fuel mass = M = DfL3 M/LLL = Df Neutron density = d = N/L3 = DfCTn/h = 7.5⋅1018 neutrons/meter3
The probability that an afterburner nucleus fissions is:
Fission cross section = A = 4801 barns Neutrons/meter2 = F = N/L2 = DfCTnL/h = 7.5⋅1018 neutrons/meter2 Fission probability = P = FA = 3.6⋅10-6
The energy/mass generated by the afterburner fissions is:
Fission energy = E = 175.8 MeV Californium-251 mass = m =4.17⋅10-25 kg Fission energy/mass = e = PE/m = 243 MJoules/kg
In a conventional thermal hydrogen rocket, atomic hydrogen exhaust has a speed of 13 km/s and an energy/mass of 84 MJoules/kg. The energy/mass of the afterburner fuel is larger than that of conventional exhaust.
Fission has a larger energ/mass than any radioactive isotope. Neutron triggering can produce larger energy/mass than fission, energy/mass than fission are is the neutron-triggered of boron-10.
Boron-10 + Neutron -> Lithium-7 + Alpha + 2.3 MeV 3837 barns Lithium-7 + Neutron -> Lithium-8 + Gamma + 2.03 MeV .0454 barns Lithium-8 -> Beryllium-8 + Beta +12.97 MeV .84 seconds Beryllium-8 -> Alpha + Alpha + 3.12 MeV 82 nanoseconds Beryllium-9 + Neutron -> Beryllium-10 + 6.81 MeV .010 barns Beryllium-10 + Neutron -> Beryllium-11 + .50 MeV .001 barns Beryllium-11 -> Boron-11 + Beta + 11.51 MeV 13.8 seconds Boron-11 + Neutron -> Boron-12 + 3.37 MeV .007 barns Boron-12 -> Carbon-12 + 13.37 MeV 20 milliseconds Carbon-12 + Neutron -> Carbon-13 + 4.95 MeV Carbon-13 + Neutron -> Carbon-14 + 8.18 MeV Carbon-14 + Neutron -> Carbon-15 Carbon-15 -> Nitrogen-15 2.45 seconds
The total energy is
Source Cross section Energy Energy/Mass
barns MeV MeV/nucleon
Hydrogen-1 .333 2.23 2.23 Gamma
Plutonium-239 748 211.5 .885 Fission fragments + Neutrons
Curium-250 220 .88 Fission fragments + Neutrons
Lithium-6 940 4.78 .797 Alpha + Tritium
Helium-3 5320 .764 .255 Proton + Tritium
Boron-10 3837 2.3 .230 Alpha + Lithium-7
Boron-10 chain 20 2
Lithium-6 39 7.25 Gamma
Californium-251 4801 Fission fragments
Gadolinium-157 254000 7.9 - 1 Gamma
Americium-242m 6400 220 .241 Fission fragments
Polonium-210 5.41 .0196 1 .106 Alpha + Lead-206
Boron-10 303 11.45 - 1 Gamma
Lithium-7 .0454 2.03 - 1 Gamma .84 seconds
Lithium-8 16.10 Beta
Beryllium-8 .0918 Alpha
Hydrogen-1 2.23 2.23 .333 Hydrogen-2 + Gamma
Fission Pu-239 211.5 .88 748 Fission fragments
Lithium-6 4.78 .80 940 Alpha + Tritium
Helium-3 .764 .26 5320 Hydrogen-3 + Proton
Boron-10 2.31 .23 3837 Lithium-7 + Alpha
Helium-3 20.58 6.86 5320 Hydrogen-4 + Gamma
Lithium-6 chain 22.90 3.82 .0454 Alpha + Tritium + Gamma
Lithium-7 18.12 2.59 .0454 Alpha + Alpha + Beta + Gamma
Boron-10 chain 20.42 2.04 .0454 Alpha + Alpha + Alpha + Beta + Gamma
Li-6 + H-2 .23
Beryllium-7 .862 .123
Hydrogen-3 .0186 .0062 0 Helium-3 + Beta
1 MeV/Nucleon = 96.5 TJoules/kg
Helium-3 20.58 6.86 5320
Hydrogen-2 6.76 3.38 .000519
Beryllium-7 18.90 2.70 Beta decay .862 MeV, .146 years
Lithium-7 chain 18.12 2.59 .0454
Hydrogen-1 2.23 2.23 .333
Boron-10 chain 20.42 2.04
Lithium-6 4.78 .80 940
Beryllium-9 6.81 .76
Carbon-12 4.95 .41
Boron-10 2.3 .23 3837
Lithium-6 7.25 39 gamma
Beryllium-8
Beryllium-10 .502
Boron-10 11.45 303 Gamma
Boron-11 3.37
Boron-10 chain 20.42 2.04
Hydrogen-1 2.23 2.23 .333
Hydrogen-2 6.76 3.38 .000519
Hydrogen-3 .000006
Helium-3 20.58 6.86 5320
Helium-4 0
Lithium-6 4.78 .80 940
Lithium-6 7.25 39 gamma
Lithium-7 2.03 .0454
Beryllium-7 18.90 Beta decay .862 MeV, .146 years
Beryllium-8
Beryllium-9 6.81 .010
Beryllium-10 .502 1.39 million years
Boron-10 2.3 3837
Boron-10 11.45 303 Gamma
Boron-11 3.37
Carbon-12 4.95
Carbon-13 8.18 .0005
Nitrogen-14 10.83
Nitrogen-15 2.49
Oxygen-16 4.14 .0001
Oxygen-17 8.04
Oxygen-18 3.96
Fluorine-19 6.60
Source Trigger Cross section Energy Energy/Mass Fragment Escape Fragment Decay output
prowess barns MeV MeV/nucleon momentum number energy
MeV⋅barn/nucleon efficiency efficiency
Helium-3 1350 5320 .764 .255 .188 2 .287 Proton + Tritium
Lithium-6 749 940 4.78 .797 .245 2 2.34 Alpha + Tritium
Boron-10 883 3837 2.3 .230 .231 1 .531 Alpha + Lithium-7
Boron-10 347 303 11.45 - 1 Gamma
Lithium-6 47 39 7.25 - 1 Gamma
Lithium-7 .0454 2.03 - 1 Gamma .84 seconds
Hydrogen-1 .7 .333 2.23 2.23 - 1 Gamma
Lithium-8 16.10 Beta
Beryllium-8 .0918 Alpha
Decay energy = E = 4.78 MeV Neutron capture cross section = A = 940 barns Lithium-6 mass = M =9.96e-27 kg Lithium-6 density
Neutrons/s/kg years
Californium-252 2.3e15 2.65
Plutonium-240 1.0e6 6600
The structural materials with the highest melting points are:
Melt Boil Density
Kelvin Kelvin g/cm3
Ta Hf carbide 4263 14.8 Ta4HfC5
Hafnium carbide 4201 12.2 HfC
Tantalum carbide 4150 15 TaC
Niobium carbide 3881 7.82 NbC
Zirconium carbide 3805 5370 6.73 ZrC
Carbon (graphite) 3800 2.15 C
Tungsten 3695 6203 19.2 W
Tantalum 3290 16.7 Ta
Tungsten carbide 3103 6270 15.6 WC
Appendix: Expanded table of melting points
Melt Density
Kelvin
Uranium 1405 19.1 2.42 46.2
Plutonium 912 19.8 2.87 56.8
Americium 1449 12 3.26 39.1
Curium 1613 13.5 3.83 51.7
Berkelium 14
Californium 1173 15.1 4.1 61.9
Einsteinium 8.84 4.2 37.1
Fermium
UO2 3138 10.97
UC 2620 13.6 12.9 2.42 31.2
UB2 2700 12.7 11.6
PuO2 3017 11.5
PuC 1931 13.5
Pu2C3 2293 12.7
PuC2 2513 10.9
AmO2 11.7
Cm2O3 2538
Metal-oxides usually have a higher melting point than the corresponding metal.
Uranium and plutonium are usually used in oxide form in nuclear reactors for their
high melting points.
The melting points of nuclear materials are:
Metal Metal-oxide Metal-carbide Metal-oxide
melt melt melt formula
Kelvin Kelvin Kelvin
Beryllium 1560 2780 2370 BeO
Cobalt 1768 2206 - CoO
Strontium 1050 2804 - SrO
Zirconium 2128 3805
Caesium 302 763 - Cs2O
Molybdenum 2896 1370 2960 MoO2
Promethium 1315 2573 Pm2O3
Europium 1099 2620 Eu2O3
Tungsten 3695 1970 3103
Polonium 527 773 PoO2
Radium 973
Uranium 1405 3138 2620 UO2
Plutonium 912 3017 PuO2
Americium 1449 AmO2
Curium 1613 Cm2O3
Californium 1173 CfO2
Cooling governs the power/mass ratio of the power source. The power/mass of a blackbody radiator is:
Stefan-Boltzmann contant = B = 5.67⋅10-8 Watts/m2/Kelvin4
Surface temperature = T
Blackbody power/area = a = BT4
Radiator mass/area = Q = 12 kg/meter
Radiator power/mass = p = BT4/Q
Temperature Power/Area Power/Mass
Kelvin kWatts/kg kWatts/kg
300 .46 .038
400 1.45 .121
500 3.54 .295
1000 56 4.725
1500 287 24
2000 907 76
2500 2210 185
3000 4590 383
Heat is transferred from the reactor to the propellant by blackbody radiation. Hydrogen is largely transparent to this radiation and so solid microparticles are added to absorb the radiation. TaC and HfC are typically used because they have the highest melting temperature of known materials.
The efficiency of a Carnot engine is:
Hot reservoir temperature = T Cold reservoir temperature = t Carnot efficiency = e = (1-t/T)
The hot reservoir temperature should be as large as possible and is govermed by the maximum temperature of the reactor materials. The choice of cold reservoir temperature is a tradeoff between thermal efficiency and power/mass. A large cold-reservoir temperature gives low efficiency a high power/mass. A small cold-reservoir temperature gives high efficiency and low power/mass.
For a fission rocket, the challenge is power. Energy is abundant and so efficiency isn't an issue. Fission thermal rockets typically chose a large value for the cold reservoir temperature to maximize power/mass.
Joules/kg/Kelvin
Hydrogen atom 12400
Hydrogen molecule 14300
Helium 5190
Lithium 3580
Beryllium 1820
Tungsten 132
Uranium 116
Thickness = X Meters Temperature differential = T Kelvin Thermal conductivity = C Watts/Kelvin/meter Heat flux = F = CT/X = T/B Watts/meter2
Melting points for oxides, carbides, nitrides, and borides:
Element Oxide Carbide Nitride Boride Hydride Fluoride Heat cap
Kelvin Kelvin Kelvin Kelvin Kelvin Kelvin Kelvin KJoules/kg
Hydrogen - 14.30
Helium 5.19
Lithium 454 1711 823 3.58
Beryllium 1560 2780 2370 2470 523 1.82
Boron 2349 723 3036 3246 108
Carbon 3800 - .709
Oxygen - 273
Sodium 371 1.23
Lead .129
Magnesium 923 3125 ? 1100 600 1.02
Aluminum 933 2345 1770 423 .897
Silicon 1687 1986 3100 88
Sulfur 222
Scandium 1814 unstable
Titanium 1941 2116 3430 3200 3500 623
Vanadium 2183 963 3080 2320 2723*
Chromium 2180 2708 2168 2443*
Manganese
Iron 1811 996 1570
Cobalt 1768 2206 1733*
Nickel 1728 1398*
Copper 1358
Zinc unstable
Selenium 239
Strontium 1050 2804 1470 2508
Zirconium 2128 2988 3805 3225 3323* 1070
Niobium 2750 2188 3881 2846 3323* NbO2
Molybdenum 2896 1370 2960 2643* 291
Technetium 2430 393 311
Ruthenium 2607 1470 exist 327
Rhodium 2237 1370 343
Palladium 1828
Silver 1234
Cadmium 594
Tin unstable
Tellurium 234
Xenon 224
Caesium 302 763
Lanthanum 2480
Gadolinium 1585 2690
Samarium 1345 2608
Lutetium 1925 3675
Hafnium 2506 4201 3520
Tantalum 3290 2145 4150 3360 3373
Tungsten 3695 1970 3103 exist 2943 276
Rhenium 3459 1273 dne 2670 292
Osmium 3306 773 future dne exist 307
Iridium 2719 1370 317
Platinum 2041 335
Lead unstable .129
Bismuth unstable
Radium 973
Element Oxide Carbide Nitride Boride Hydride Fluoride Heat cap
Kelvin Kelvin Kelvin Kelvin Kelvin Kelvin Kelvin KJoules/kg
Actinium 3471
Thorium 5061
Protactinium 4300
Uranium 1405 3138 2620 1170 2700 337 4404
Neptunium 328 4273
Plutonium 912 3017 325 3505
Americium 1449 2478 2880
Curium 1613 1992 3383
Californium 1173 2023 1743
Einsteinium 1133 1269
Fermium 1800
Diamond
Quartz
Graphite
Corundum 2317
Lead unstable .129 1.46 11.34
Element Oxide Carbide Nitride Boride Hydride Fluoride Heat cap Heat cap Density Boil
Kelvin Kelvin Kelvin Kelvin Kelvin Kelvin Kelvin KJoules/kg KJoules/litre gram/cm3
Melt Boil Density
Kelvin Kelvin g/cm3
HfCN 4400 HfCN
TaHf carbide 4263 14.8 Ta4HfC5
Hafnium carbide 4201 12.2 HfC
Tantalum carbide 4150 ~15 TaC
Niobium carbide 3881 7.82 NbC
Zirconium carbide 3805 5370 6.73 ZrC
Carbon (graphite) 3800 2.15
Tungsten 3695 6203 19.2
Hafnium diboride 3520 10.5 HfB2
Zirconium diboride 3519 6.08 ZrB2
Titanium diboride 3500 4.52 TiB2
Rhenium 3459 21.0
Titanium carbide 3430 5090 4.93 TiC
Tantalum diboride 3373 TaB2
Tantalum nitride 3360 14.3 TaN
Osmium 3306 5285 22.6
Tantalum 3290 16.7
Boron nitride 3246 2.1 BN
Titanium nitride 3200 5.22 TiN
Uranium oxide 3138 10.97 UO2
Magnesium oxide 3125 3870 3.6 MgO
Tungsten carbide 3103 6270 15.6 WC
Silicon carbide 3100 3.16 SiC
Vanadium carbide 3080 5.77 VC
Boron carbide 3036 3770 2.52 B4C
Plutonium oxide 3017 3070 11.5 PuO2
Molybdenum carbide 2960 8.90 MoC
Tungsten boride 2943 W2B
Tungsten boride 2928 WB
Molybdenum 2896 10.3
Strontium oxide 2804 3470 4.70 SrO
Beryllium oxide 2780 4170 3.01 BeO
Niobium 2750 8.57
Iridium 2719 22.6
Uranium diboride 2700 12.7 UB2
Uranium carbide 2620 13.63 UC
Ruthenium 2607 12.4
Strontium hexaboride2508 3.39 SrB6
Hafnium 2506 13.3
Lanthanum hexaboride2480 4.72 LaB6
Beryllium nitride 2470 2510 2.71 Be3N2
Technetium 2430 11
Beryllium carbide 2370 1.9 Be2C
Boron 2349 2.34
Aluminum oxide 2345 3250 3.99 Al2O3
Vanadium nitride 2320 6.13 VN
Sapphire 2300 4.0 Al2O3
Rhodium 2237 12.4
Vanadium 2183 6.0
Chromium 2180 7.15
Chromium carbide 2168 4070 6.68 Cr3C2
Tantalum oxide 2145 8.13 Ta2O5
Zirconium 2128 6.52
Cobalt oxide 2206 6.44 CoO
Titanium oxide 2116 3245 4.23 TiO2
Quartz 1986 2.65 SiO2
Tungsten oxide 1970 10.8 WO2
Titanium 1941 4.51
Palladium 1828 12.0
Scandium 1814 2.98
Iron 1811 7.86
Aluminum carbide 1770 2.93 Al4C3
Cobalt 1768 3200 8.90
Nickel 1728 8.91
Lithium oxide 1711 2870 2.01 Li2O
Silicon 1687 2.33
Curium 1613 3383 13.5
Iron boride 1570 7.15 FeB
Beryllium 1560 1.85
Americium 1449 2880 12
Uranium 1405 4404 19.1
Molybdenum dioxide 1370 6.47
Copper 1358 8.96
Silver 1234 10.5
Californium 1173 1743 15.1
Uranium nitride 1170 11.3 UN
Magnesium diboride 1100 2.57 MgB2
Strontium 1050 1650 2.64
Iron carbide 996 Fe3C
Radium 973 2010 5.5
Aluminum 933 2.70
Magnesium 923 1.74
Plutonium 912 3505 19.82
Polonium 527 1235 9.3
Lithium carbide 823 1.3 Li2C2
Caesium oxide 763 4.65 Cs2O
Cadmium 594 8.65
Lithium 454 .53
Sodium 371 .97
Caesium 302 944 1.93
Tantalum hydride 15.1 TaH
Hafnium hydride 11.4 HfH2
Zirconium hydride 1070 5.6 ZrH2
Power/Mass Half life Decay Melt Decay Side Distance Potholes
Watt/kg year MeV mode
Rhenium-184 .104 5869 e+ < 1
Tungsten-188 .191 .349 3695 3695 6203 Metal e- > 2
Hafnium-181 .116 2506 4201 4876 Carbide e- > 1
Hafnium-175 .192 2506 4201 4876 Carbide e+ > 1
Osmium-185 .256 EC > 1
Tungsten-181 9546 .332 .1877 3695 3695 6203 Metal EC
Rhenium-184m .463 .188 IT or e+ < 1
Rhodium-102 67003 .567 1.268 2237 3968 e+ or e-
Ruthenium-106 1116 1.018 .0394 2607 4423 e-
Tantalum-179 991 1.82 .1056 3290 4150 5731 Carbide EC < 1
Hafnium-172 3210 1.87 .338 2506 4201 4876 Carbide EC < 2
Rhodium-101 4967 3.3 .542 2237 3968 EC < 2
Osmium-194 6.02 2.33 3306 5285 e- > 2 pothole 30.1 hours
Niobium-93m 16.13 .03077 2750 3881 5017 Carbide Gamma = 0
Hafnium-178m2 1401 31 2.446 2506 4201 4876 Carbide Gamma = 0
Platinum-193 50 .0568 EC > 1
Uranium-232 1035 68.9 5.414 1405 3138 4404 Oxide Alpha
Niobium-91 680 2750 3881 5017 Carbide < 2
Plutonium-238 818 87.7 5.59 912 3017 3505 Oxide Alpha
Plutonium-241 4315 14.4 4.90 912 3017 3505 Oxide Alpha
Curium-243 2666 29.1 6.169 1613 1992 3383 Oxide Alpha
Curium-244 4014 18.1 5.80 1613 1992 3383 Oxide Alpha
Curium-250 241 8300 5.17 1613 1992 3383 Oxide SF or Alpha
Cobalt-60 27300 5.27 2.82 1768 2206 3200 Oxide e-, Gamma
Beryllium-7 .146 2742 EC < 2
Californium-252 58470 2.64 6.12 1173 2023 1743 Oxide Alpha or SF
Californium-250 5779 13.1 6.02 1173 2023 1743 Oxide Alpha or SF
Beryllium-7 2577000 .146 .862 1560 2780 2742 Oxide EC
Titanium-44 295 63 .268 1941 3430 3560 Carbide EC
Titanium-60 60.0 EC <
Niobium-93m 16.13 .03077 IT > 0
Ruthenium-106 1.023 e- > 2 105 is 4.44 hours
Rhodium-102 .567 e+ or e-
Rhodium-102m 3.742 .1408 e+
Vanadium-49 .901 EC <
Zirconium-95 .175 > 1
Lutetium-173 1.37 EC <
Iridium-192 .202 e- or EC > 1
Lutetium-174 3.31 3675 e+ < 1
Lutetium-174m1 .389 .171 3675 IT < 1
Lutetium-177 .0182 e- > 1
Lutetium-177m3 .439 .970 e- or IT > 1
Melt Neutron capture
Kelvin barn
Ta Hf carbide 4263 60
Hafnium carbide 4201 104
Tantalum carbide 4150 20
Niobium carbide 3881 1.15
Zirconium carbide 3805 .184
Diamond 3800 .0035
Tungsten 3695 18.3
Rhenium 3459 89.7
Osmium 3306 15
Tantalum 3290 20.6
Tungsten carbide 3103 18.3
Iridium 2719 425
Hafnium 2506 104
Molybdenum 2896 2.6
Niobium 2750 1.15
Ruthenium 2607 2.56
Technetium 2430 20
Rhodium 2237 145
Vanadium 2183 5.08
Chromium 2180 3.1
Zirconium 2128 .184
Platinum 2041 .96
Titanium 1941 6.09
Lutetium 1925 84
Palladium 1828
Fermium 1800
Curium 1613
Americium 1449
Uranium 1405
Californium 1173
Einsteinium 1133
Plutonium 912
 |
|---|