




 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
 |
 |
 |
.jpg) |
|---|---|---|---|---|
 |
|---|
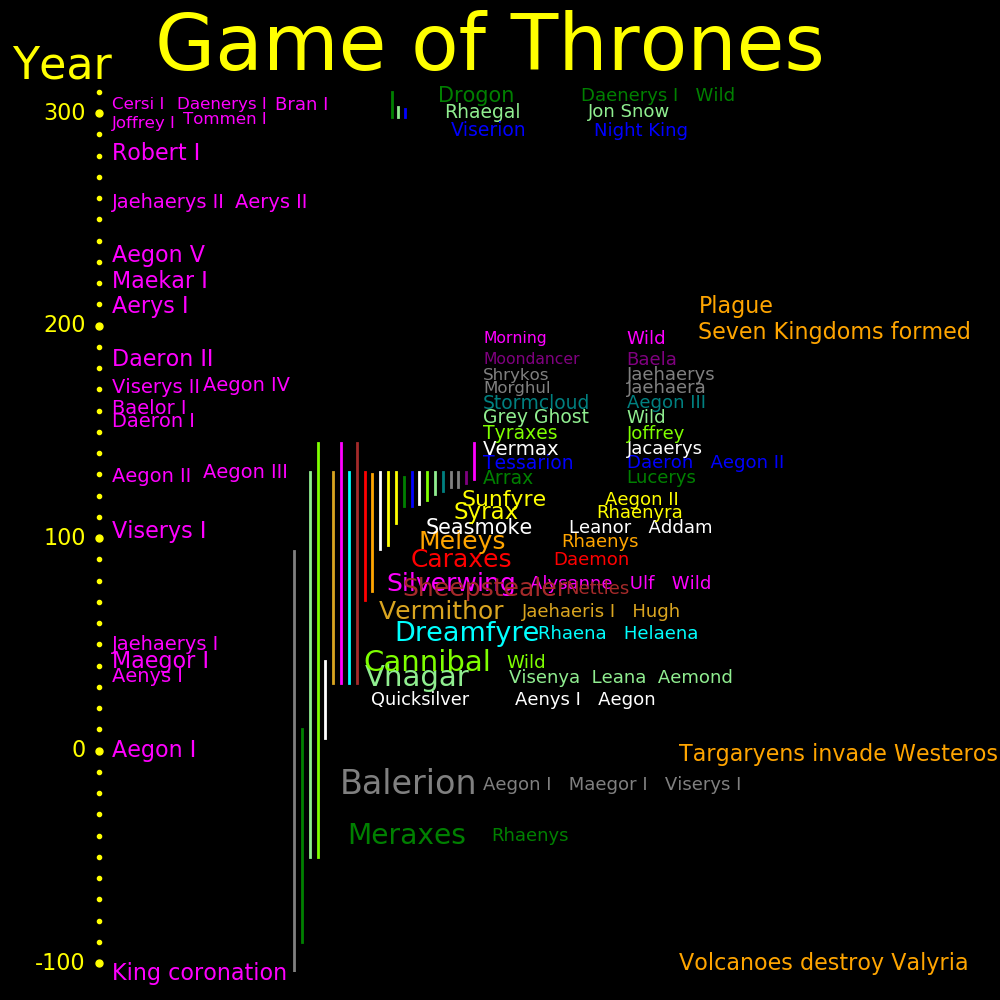
Dragon Birth Death Size Riders Balerion -126 94 1 Aegon I, Maegor I, Viserys I Died of old age Meraxes -90 10 .75 Rhaenys Killed by an arrow Vhagar -50 131 .8 Visenya, Leana Velaryon, Aemond Died in fight with Caraxes Cannibal -50 145 .8 Riderless Lived past the Dance of Dragons Quicksilver 6 42 .25 Aenys I, Aegon Killed by Balerion Dreamfyre 32 131 .7 Rhaena, Helaena Killed in the dragonpit by a mob Vermithor 32 131 .6 Jaehaerys I, Hugh Hammer Died in the Dance of Dragons from fighting Seasmoke Silverwing 32 145 .6 Alysanne, Ulf the White, Riderless Fought in the Dance of Dragons and was the only survivor Sheepstealer 32 145 .6 Nettles Lived past the Dance of Dragons Caraxes 71 131 .6 Daemon Died in fight with Vhagar Meleys 75 130 .6 Rhaenys Killed by Sunfyre and Vhagar Seasmoke 95 131 .4 Leanor, Addam Killed by Vermithor in the Dance of Dragons Syrax 97 131 .5 Rhaenyra Killed in the dragonpit by a mob Sunfyre 107 131 .45 Aegon II Died of injuries from dragon fights Tessarion 115 131 .3 Daeron Aegon II Died in the Dance of Dragons from fighting Seasmoke Vermax 116 131 .35 Jacaerys Killed by an arrow Grey Ghost 121 131 .3 Riderless Killed by Sunfyre Stormcloud 122 131 .3 Aegon III Died naturally Arrax 115 129 .3 Lucerys Velaryon Killed by Vhagar Tyraxes 118 131 .3 Joffrey Killed in the dragonpit by a mob Moondancer 126 131 .15 Baela Killed by Sunfyre Morghul 124 131 .2 Jaehaera Killed in the dragonpit by a mob Shrykos 124 131 .2 Jaehaerys Killed in the dragonpit by a mob Morning 128 145 .15 Rhaena Lived past the Dance of Dragons Drogon 298 .4 Daenerys Alive after "Game of Thrones" Rhaegal 298 303 .3 Jon Snow Killed by an arrow Viserion 298 302 .3 Night King Killed by the Night King. Undead version killed by Arya
# Years Start End Dragon Nickname
Aegon I 37 0 37 Balerion Conquerer
Aenys I 5 37 42 Quicksilver
Maegor I 6 42 48 Balerion Cruel
Jaehaerys I 55 48 103 Vermithor Conciliator
Viserys I 26 103 129 Balerion
Aegon II 2 129 131 Sunfyre
Rhaenyra I 0 131 131 Syrax
Aegon II 0 131 131 Sunfyre
Aegon III 26 131 157 Stormcloud Dragonbane The last dragon died during his reign
Daeron I 4 157 161 Young Dragon
Baelor I 10 161 171 Beloved
Viserys II 2 171 173
Aegon IV 11 172 184 Unworthy
Daeron II 25 184 209 Good
Aerys I 12 209 221
Maekar I 12 221 233
Aegon V 25 233 258 Unlikely
Jaehaerys II 3 258 261
Aerys II 18 262 280 Mad King
Robert I 18 280 298
Joffrey I 3 298 301
Tommen I 2 301 303
Cersi I 2 303 305
Daenerys I 0 305 305 Drogon Breaker of Chains
Bran I 305 Broken
At the start of the war, Aegon II had 6 dragons and Rhaenyra had 12. After the war, Aegon II had 2 and Rhaenyra had none. Rhaenyra was toasted by Aegon II's dragon Sunfyre.
The war was precipitated by the death of King Viserys I. The Westeros lords were split on the successor, with some favoring Aegon II and some favoring Rhaenyra. Rhaenyra had more dragons and decided to fight for the throne. She managed the war poorly and lost all her dragons.
At the end of the war, Sunfyre died and Silverwing went rogue. At this point, there were 4 dragons left in the world and the Targaryens controlled none of them. They were all rogue.
The war started when Aemond and Vhagar killed Lucerys and Arrax. The killing was unwise, because the likely result is war and the death of dragons. The war weakened the Targaryen house.
Sunfyre killed 3 dragons, a mob killed 4 dragons, and 3 dragons died in the Dance of Dragons.
Westeros is a balance of power between Targaryens, Lords, and the Faith of the Seven. The balance of power disintegrated.
A synergistic relationship is possible between Targaryens and lords. Targaryens have the virtue of dragons, which protect the realm from invasion. Lords have the virtue that they can manage the people. An example of a synergistic balance of power is that lords let the Targaryens have the throne, and that the Targaryens respect the autonomy of lords.
The lords need to be unified to keep the Targaryens in check. A Targaryen king should require the support of lords. Once Aegon II and Rhaenyra started fighting, lords should have demanded that neither be monarch, and the lords should have chosen a different Targaryen.
Faction Dragon Rider Aegon II Vhagar Aemond Died in fight with Caraxes Aegon II Sunfyre Aegon II Died of injuries from dragon fights Aegon II Silverwing Alysanne, Ulf, Riderless Survived the Dance of Dragons Aegon II Vermithor Jaehaerys I, Hugh Died of injuries from fighting Seasmoke in the Dance of Dragons Aegon II Tessarion Daeron Died of injuries from fighting Seasmoke in the Dance of Dragons Aegon II Dreamfyre Helaena Killed in the dragonpit by a mob Rhaenyra Meleys Rhaenys Killed by Sunfyre and Vhagar Rhaenyra Moondancer Baela Killed by Sunfyre Rhaenyra Grey Ghost Killed by Sunfyre Rhaenyra Caraxes Daemon Died in fight with Vhagar Rhaenyra Arrax Lucerys Killed by Vhagar Rhaenyra Vermax Jacaerys Killed by an arrow Rhaenyra Seasmoke Leanor, Addam Killed by Vermithor in the Dance of Dragons Rhaenyra Syrax Rhaenyra Killed in the dragonpit by a mob Rhaenyra Tyraxes Joffrey Killed in the dragonpit by a mob Rhaenyra Morghul Killed in the dragonpit by a mob Rhaenyra Shrykos Killed in the dragonpit by a mob Rhaenyra Sheepstealer Nettles Seceded from Rhaenyra's command
 |
|---|
 |
|---|
 |
|---|
 |
|---|
 |
|---|
 |
 |
|---|---|
Valyrian steel is a fictional substance from "Game of Thrones" that is stronger, lighter, and harder than steel. The only elements that qualify are beryllium, titanium, and vanadium, none of which were known in Earth history until the 18th century. Valyrian steel could be of these elements, an alloy, or a magical substance. According to George Martin, magic is involved.
The fact that it is less dense than steel means that it can't be a fancy form of steel such as Damascus steel or Wootz steel. Also, fancy steel loses its special properties if melted and hence cannot be reforged, whereas Valyrian steel swords can be reforged.
In Earth history, the first metal discovered since iron was cobalt in 1735. This launched a frenzy to smelt all known minerals and most of the smeltable metals were discovered by 1800. Then the battery and electrochemstry were discovered in 1800 and these were used to obtain the unsmeltable metals, which are lithium, beryllium, magnesium, aluminum, titanium, vanadium, niobium, and Uranium. Almost all of the strong alloys use these metals, and so the Valyrians must have used either electrochemistry or magic to make Valyrian steel.
The following metals and alloys are both stronger and lighter than steel and could hypothetically be Valyrian steel.
Yield Density Strength/Density
strength (g/cm3) (GJoule/kg)
(GPascal)
Beryllium .34 1.85 .186
Aluminum + Be .41 2.27 .181
LiMgAlScTi 1.97 2.67 .738
Titanium .22 4.51 .050
Titanium + AlVCrMo 1.20 4.6 .261
Vanadium .53 6.0 .076
AlCrFeCoNiTi 2.26 6.5 .377
AlCrFeCoNiMo 2.76 7.1 .394
Steel .25 7.9 .032 Iron plus carbon
Copper .12 9.0 .013
"Yield strength" is the maximum pressure a material can sustain before
deforming. "Strength/Density" is the strength-to-weight ratio.
Steel is stronger and lighter than copper.
Petyr Baelish: Nothing holds an edge like Valyrian steel.
Tyrion Lannister: Valyrian steel blades were scarce and costly, yet thousands remained in the world, perhaps two hundred in the Seven Kingdoms alone.
George Martin: Valyrian steel is a fantasy metal. Which means it has magical characteristics, and magic plays a role in its forging.
George Martin: Valyrian steel was always costly, but it became considerably more so when there was no more Valyria, and the secret of its making were lost.
Ned Stark's stord "Ice" is melted down and reforged into two smaller swords, "Oathkeeper" and "Widow's Wail". This rules out Valyrian steel being Wootz steel because Wootz steel loses its special properties when reforged.
Appearances of Valyrian steel in Game of Thrones:
Name Owner
Sword Longclaw Jon Snow
Sword Heartsbane Samwell Tarly
Dagger Arya
Sword Ice Eddard Stark Reforged into Oathkeeper and Widow's Wail
Sword Oathkeeper Brienne of Tarth
Sword Widow's Wail The Crown
Sword Lady Forlorn Ser Lyn Corbray
Sword Nightfall Ser Harras Harlow
Sword Red Rain Lord Dunstan Drumm
Arakh Caggo
Armor Euron Greyjoy
Horn Dragonbinder The Citadel of The Maesters
Some Maesters carry links of Valyrian steel, a symbol of mastery of the highest arts.
 |
 |
 |
|---|---|---|
The Dawn sword was forged from meteorite metal, which has the composition:
Mass Yield strength Density
fraction MPascal gram/cm3
Iron .91 200 7.9
Nickel .067 480 8.9
Cobalt .0063 480 8.9
Nickel and cobalt are 2.5 times stronger than iron, and the alloy of iron, nickel, and cobalt is even stronger. Alloys are usually stronger than their constituent metals.
Dawn is described as being more reflective than steel. The reflectivity of various metals is:
Silver .98 Aluminum .97 Copper .96 Nickel .93 Steel .93
Steel and nickel have the same reflectivity. A meteorite sword has the same reflectivity as steel.
 |
 |
|---|---|
The burn rate of gasoline is limited by the supply of oxygen.
C8H18 + 12.5 O2 → 8 CO2 + 9 H2OGunpowder has oxygen in the mixture in the form of KNO3 which makes it burn faster.
3 C + S + 2 KNO3 → K2S + N2 + 3 CO2We know that wildfire contains an oxidizer otherwise it wouldn't be able to explode as it did on the show. Wildfire is made from manure, which contains KNO3.
Copper burns with a green flame. Adding copper powder to the explosive adds energy to the blast.
Three types of incendiaries are:
Gasoline: Flame spreads slowly. Needs oxygen from the air. Gunpowder: Contains oxygen. Buns faster than gasoline. Subsonic pressure wave. Plastic explosive: Pressure wave spreads supersonically as a shock.
 |
|---|
The Lightbringer sword is perpetually hot. This can achieved within the laws of physics by adding a radioactive isotope to the metal.
The composition of the sword is unknown. If it's steel then the maximum temperature is 900 Kelvin and if it's another metal the temperature could potentially be much higher. Steel loses strength as it heats and at 900 Kelvin it has half its room-temperature strength.
Typical parameters for a sword are:
Sword mass = M = DLWH = 1.2 kg Sword length = L = 1.0 meters Sword width = W = .05 meters Sword thickness = H = .003 meters Sword density = D =7900 kg/meter2
The sword temperature is determined by a balance between radioactive heating and blackbody radiation. If the sword has a temperature of 900 Kelvin then the heating power/mass is:
Sword blackbody emissivity= f = .07 For steel Stefan-Boltzmann constant = C =5.670⋅10-8 Watts/m2/Kelvin4 Blackbody power/area = f C T4 = 2604 Watts/meter2 Sword heat power/mass = p = 220 Watts/kg Sword heat power/area = ½ p D H = f C T4 = 2604 Watts/meter2 Sword total power = P = pM = 264 Watts
Many radioactive isotopes have a sufficiently high power/mass to make such a sword. The isotopes with a half life larger than 10 years and with a power/mass larger than 100 Watts/kg are:
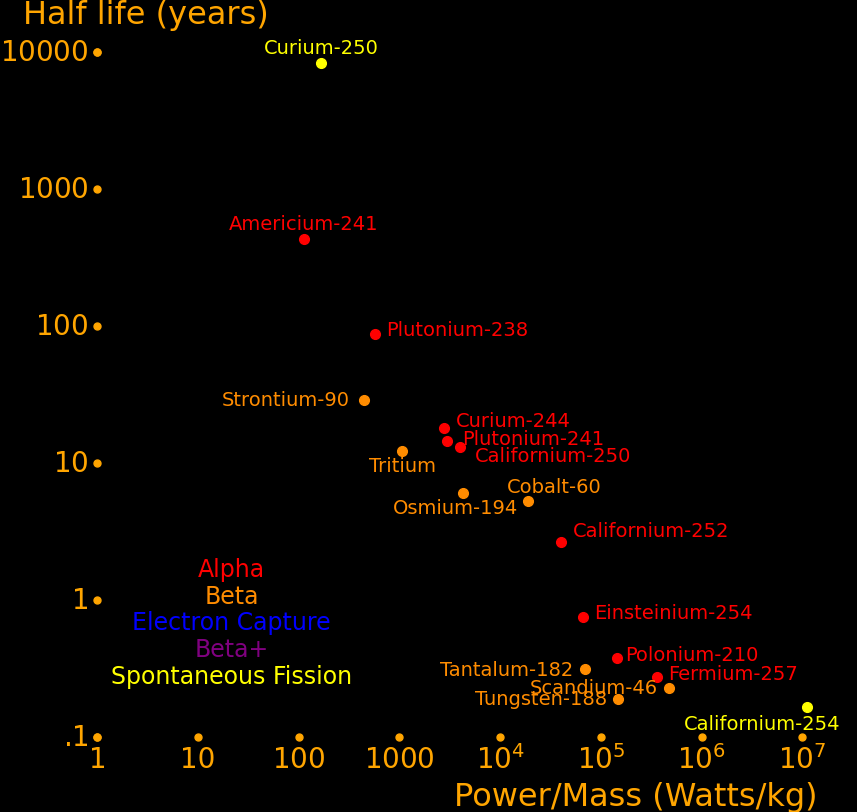 |
|---|
Power/Mass Energy/Mass Halflife Decay Decay
Watts/kg GJoules/kg years MeV mode
Californium-250 5779 2385 13.1 6.02 α or SF
Plutonium-241 4315 1961 14.4 4.90 α
Curium-244 4014 2293 18.1 5.80 α
Curium-243 2666 2449 29.1 6.169 α
Caesium-137 1736 1654 30.2 2.35 β
Hydrogen-3 1538 598 12.32 .0186 β
Europium-152 1194 509 13.5 .802 EC,β-+,γ
Uranium-232 1035 2251 68.9 5.414 α
Plutonium-238 818 2265 87.7 5.59 α
Strontium-90 648 589 28.8 .55 β
Curium-250 241 63170 8300 5.17 SF or α
Americium-241 161 2197 432 5.49 α
The sword was forged 8000 years ago and there is a question of if it would still be hot. The only isotope capable of powering the sword this long is curium-250, which has a half life of 8300 years. It has a superlatively high power/mass because it's the only isotope that decays primarily by spontaneous fission.
The metal emissivity should be as low as possible to minimize heat loss. You also want to polish the sword to minimize emissivity.
The metals with high melting points are:
Melt Density
Kelvin g/cm3
Tungsten 3695 19.2
Rhenium 3459 21.0
Osmium 3306 22.6
Tantalum 3290 16.7
Molybdenum 2896 10.3
Niobium 2750 8.57
Iridium 2719 22.6
Ruthenium 2607 12.4
Hafnium 2506 13.3
Technetium 2430 11
Boron 2349 2.34
Rhodium 2237 12.4
Vanadium 2183 6.0
Chromium 2180 7.15
Zirconium 2128 6.52
Titanium 1941 4.51
Palladium 1828 12.0
Scandium 1814 2.98
Steel 1811 7.86
Cobalt 1768 8.90
Nickel 1728 8.91
Curium 1613 13.5
The metal with the best combination of high melting point and low density is molybdenum.
The hottest the sword could be is if it's made of tungsten, which at 1700 Kelvin has half its room
temperature strength. At 1700 Kelvin it has a power/mass of 1600 Watts/kg.
The isotopes with a sufficiently large power/mass are californium-250, promethium-146, plutonium-241, curium-244, and curium-243. The one with the longest half life is curium-243, with a half life of 29 years.
 |
|---|
Speed Mass Energy
m/s kg Joules
Bow 74 .021 57
Compound bow 113 .035 223
Crossbow 75 .040 112
Slingshot 75 .0063 18
Ballista 75 5 14000
Trebuchet 40 100 80000
Cannonball 6 lb 438 2.7 261000
Cannonball 36 lb 450 16.3 1653000
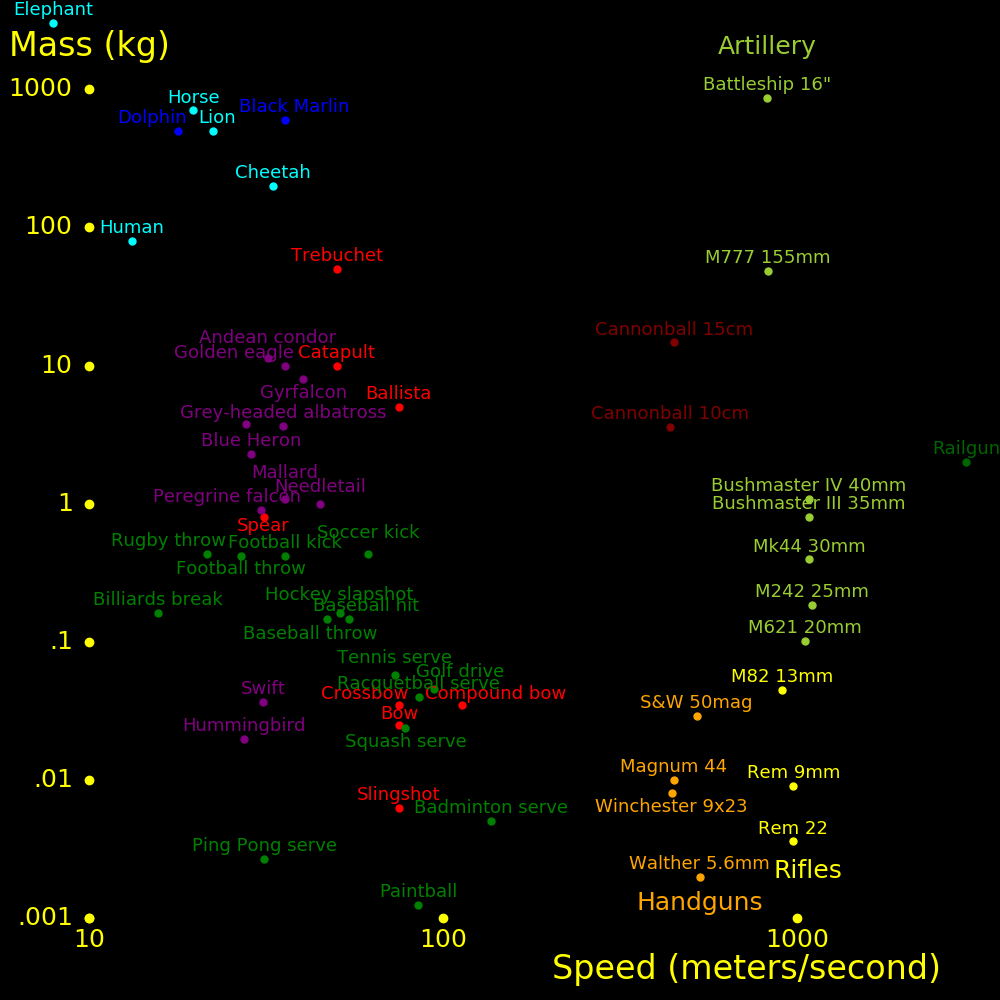
Typical parameters for a bow and arrow are:
Arrow speed = 74 meters/second Arrow mass = 20.6 g Arrow kinetic energy = 57 Joules Bow energy = 74 Joules Energy efficiency = .77 Maximum bow force =200 Newtons Bow mass = 6.5 kg Bow mass / Arrow mass =316 Arrow length = .70 meters Arrow radius = 3.63 mm Arrow wall thickness = .50 mm Arrow tip mass = 6.2 g Arrow shaft mass = 11.7 g Arrow fin mass = 1.4 g Arrow nock mass = 1.2 g Carbon fiber stiffness= 85 GPascalsData from the paper "Applications of Physics to Archery", Dr. H. O. Meyer.
Arrow mass = M Air density = D = 1.19 kg/meters2 Arrow cross section = A Air drag coefficient = C = 1.94 Arrow speed = V Air drag force = F = ½ C D A V2 Arrow acceleration = W = F/M = c V2 Air drag parameter = c = ½ C D A M-1 =.00231 meters-1 Arrow initial speed = 74 meters/second Arrow speed at .1 km = 66 meters/second
The Warwick Castle trebuchet is the largest trebuchet in the world.
Trebuchet mass = 22 tons Projectile mass = 15 kg Projectile range = 168 meters Counterweight mass =2000 kg Trebuchet height = 19 meters
For a projectile fired at 45 degrees,
Horizontal speed = v Vertical speed = v Total speed = V = 2½ v Gravity = g = 9.8 meters/second2 Flight time = T = 2 v/g Range = R = vT = V2/g
The earliest metals were gold and silver, the only ones that occur naturally in pure form.
Iron can occasionally be found as iron meteorites.
Copper was discovered around 7000 BCE by smelting copper minerals in a wood fire.
Around 3200 BCE it was found that copper is strenghened by tin, and this is called bronze.
Around 2000 BCE it was found that copper is also strengthed by zinc, and this is called brass.
The earliest metals were smeltable with a wood fire and they consist of copper, lead, silver, tin, zinc, and mercury.
They come from the following minerals:
Gold and silver were known since antiquity, but gold mining didn't start until 6000 BC, and silver smelting didn't
start until 4000 BC.
The minerals that were used by ancient civilizations to smelt metal are:
The next metal to be discovered was iron (c. 1200 BC), which requires a bellows-fed coal fire to smelt.
No new metals were discovered until cobalt in 1735. Once cobalt was discovered, it was realized that
new minerals may have new metals, and the race was on to find new minerals. This yielded
nickel, chromium, manganese, molybdenum, and tungsten.
Chromium is lighter and stronger than steel and it was discovered in 1797. It
satisfies the properties of "Valyrian steel" from Game of Thrones. There's no reason chromium couldn't have
been discovered earlier.
Coal smelting can't produce the metals lighter than chromium. For these you
need electrolysis. The battery was invented in 1799, enabling electrolysis, and
the lighter metals were discovered shortly after. These include aluminum, magnesium, titanium,
and beryllium.
Carbon fiber eclipses metals. The present age could be called the carbon age. The carbon age became
mature in 1987 when Jimmy Connors switched from a wood to a carbon racket.
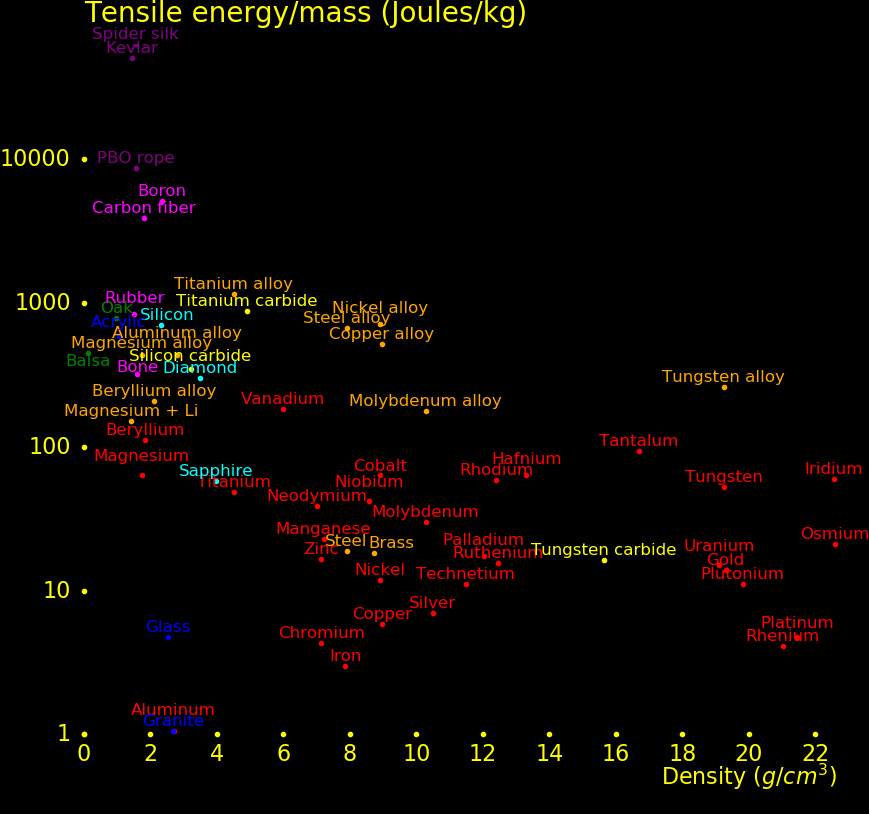
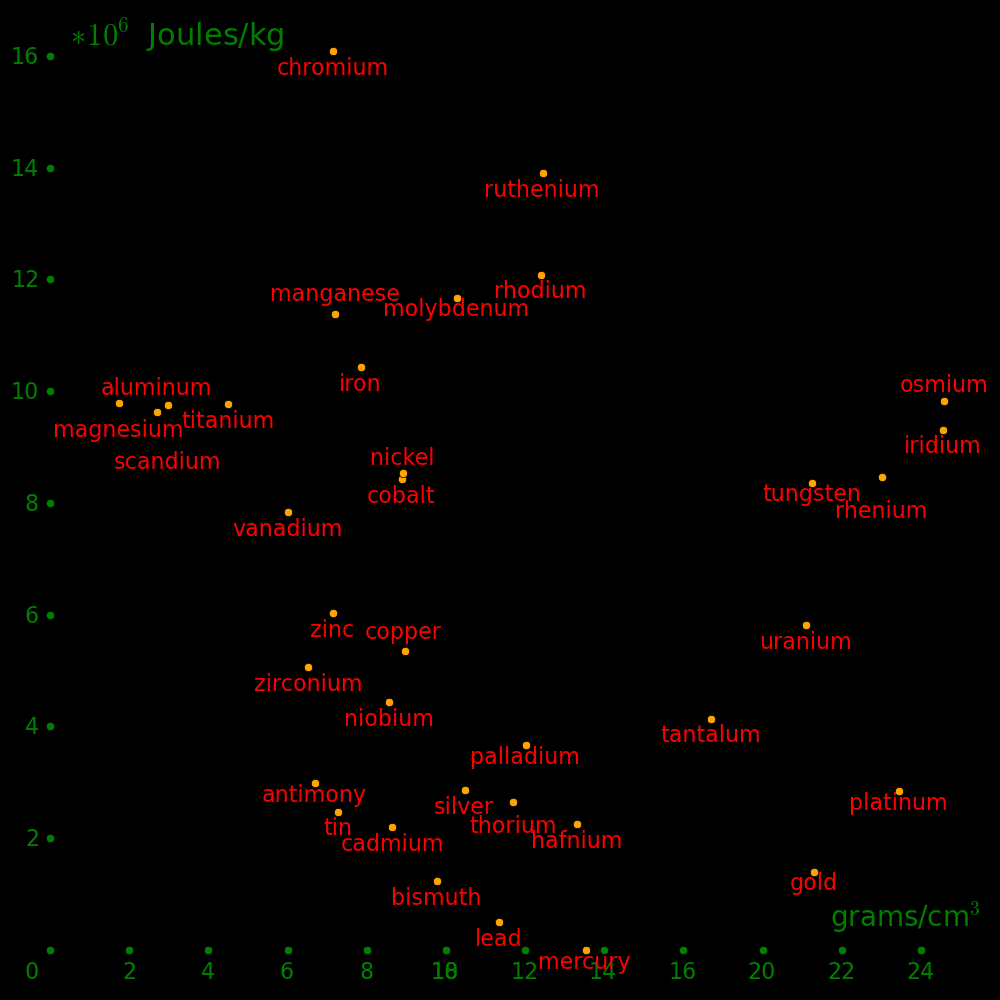
The plot shows the strength of materials.
Wood rivals alloys for strength.
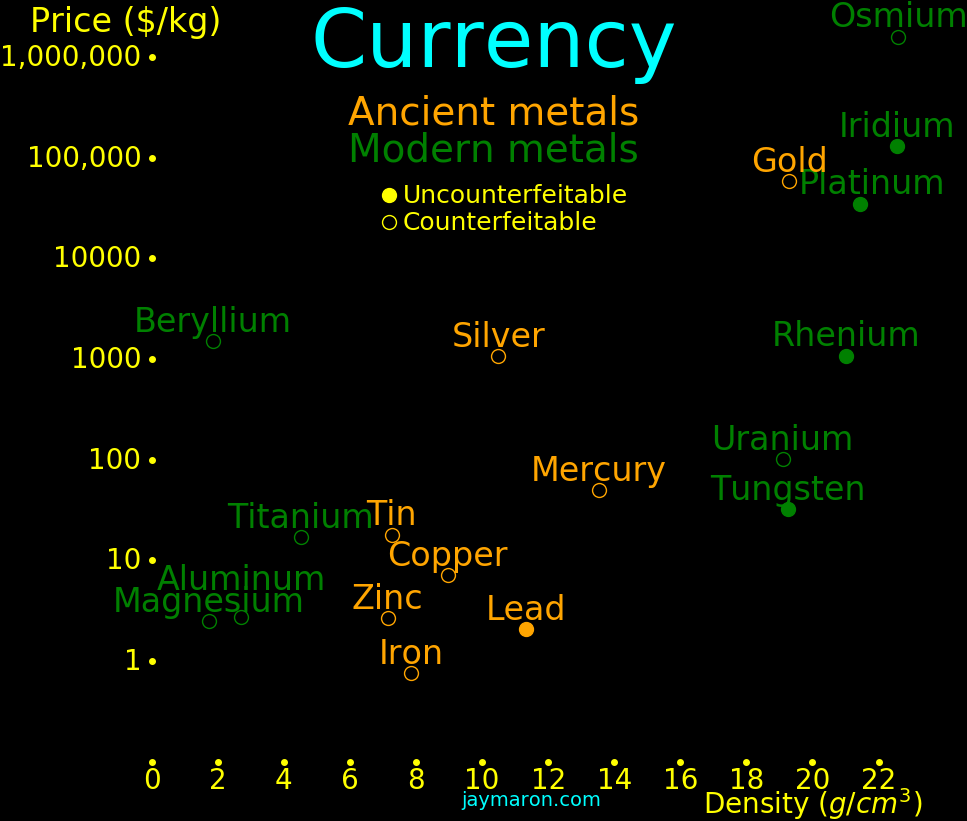
Gold was the densest element known until the discovery of platinun in 1735. It
was useful as an uncounterfeitable currency until the discovery of tungsten in
1783, which has the same density as gold. Today, we could use iridium,
platinum, or rhenium as an uncounterfeitable currency.
Prior to 1800, metals were obtained by smelting minerals, and the known metals
were gold, silver, copper, iron, tin, zinc, mercury, cobalt, manganese,
chromium, molybdenum, and tungsten. Elements to the left of chromium titanium
and scandium cant's be obtained by smelting, and neither can aluminum,
magnesium, and beryllium. They require electrolysis, which was enabled by
Volta's invention of the battery in 1799.
Prior to 1800, few elements were known in pure form. Electrolyis enabled the
isolation of most of the rest of the elements. The periodic table then became
obvious and was discovered by Mendeleev 1871. The battery launched modern
chemistry, and the battery could potentially have been invented much earlier.
Electrolysis enabled the isolation of sodium and potassium in 1807, and these were used
to smelt metals that can't be smelted with carbon.
For a metal, the stiffness is characterized by the "shear strength" and the
sword worthiness is characterized by the shear strength over the density
(the "strength to weight ratio"). For example for iron,
This plot includes all metals with a strength/density at least as large as lead,
plus mercury.
Beryllium is beyond the top of the plot.
In prehistoric times iron meteorites were the only source of metallic iron.
They consist of 90% iron and 10% nickel.
Most metals are in oxidized form. The only metals that can be found in
pure form are gold, silver, copper, platinum, palladium, osmium, and iridium.
Smelting is a process for removing the oxygen to produce pure metal.
The ore is heated in a coal furnace and the carbon seizes the oxygen from
the metal. For copper,
For iron, the oxidation state is reduced in 3 stages until the
pure iron is left behind.
The following table gives the temperature required to smelt each element with
carbon.
The farther to the right on the periodic table, the lower the smelting
temperature, a consequence of "electronegativity".
The battery was invented in 1800, launching the field of electrochemistry
and enabling the the isolation of non-carbon-smeltable elements.
Davy used electrolysis in 1807 to isolate sodium and potassium and then he used
these metals to smelt other metals. To smelt beryllium with potassium,
BeO + 2 K ↔ Be + K2O.
Titanium can't be carbon smelted because it forms the carbide Ti3C.
For an expanded discussion of smelting physics, see jaymaron.com/metallurgy.html.
Thermite is smelting with aluminum. For example, to smelt iron with aluminum,
The following table shows reactions that change the oxidation state of a metal.
"M" stands for an arbitrary metal and the magnitudes are scaled to one mole of
O2. The last two columns give the oxidation state of the metal on
the left and right side of the reaction. An oxidation state of "0" is the pure
metal and "M2O" has an oxidation state of "1".
These elements are not necessarily on the Science Olympiad list.
We list minerals by element, with the most abundant mineral for each element listed first.
![]()













.jpg)










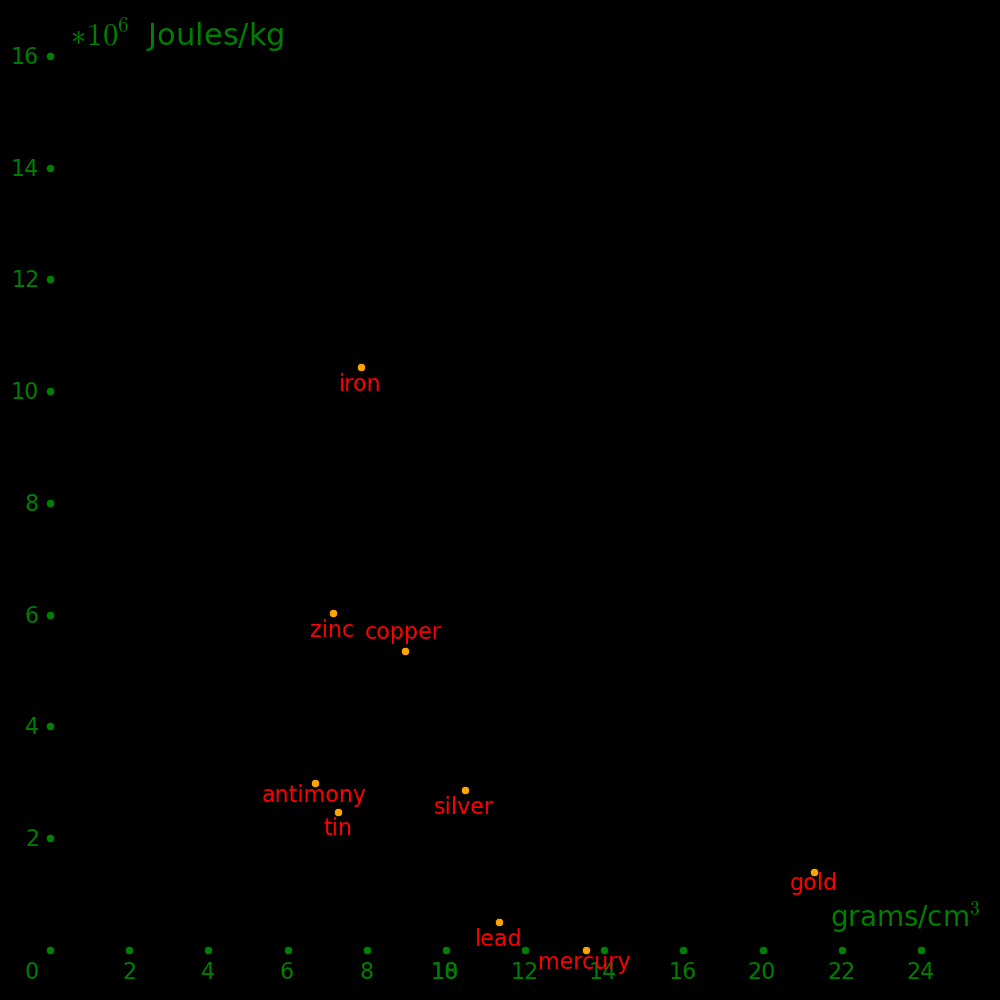
Alloys can be much stronger than pure metals.

Discovery Method of Source
(year) discovery
Carbon Ancient Naturally occuring
Gold Ancient Naturally occuring
Silver Ancient Naturally occuring
Sulfur Ancient Naturally occuring
Lead -6500 Smelt with carbon Galena PbS
Copper -5000 Smelt with carbon Chalcocite Cu2S
Bronze (As) -4200 Copper + Arsenic Realgar As4S4
Tin -3200 Smelt with carbon Calamine ZnCO3
Bronze (Sn) -3200 Copper + Tin
Brass -2000 Copper + Zinc Sphalerite ZnS
Mercury -2000 Heat the sulfide Cinnabar HgS
Iron -1200 Smelt with carbon Hematite Fe2O3
Arsenic 1250 Heat the sulfide Orpiment As2S3
Zinc 1300 Smelt with wool Calamine ZnCO3 (smithsonite) & Zn4Si2O7(OH)2·H2O (hemimorphite)
Antimony 1540 Smelt with iron Stibnite Sb2S3
Phosphorus 1669 Heat NaPO3 Excrement
Cobalt 1735 Smelt with carbon Cobaltite CoAsS
Platinum 1735 Naturally occuring
Nickel 1751 Smelt with carbon Nickeline NiAs
Bismuth 1753 Isolated from lead
Hydrogen 1766 Hot iron + steam Water
Oxygen 1771 Heat HgO
Nitrogen 1772 Isolated from air
Manganese 1774 Smelt with carbon Pyrolusite MnO2
Molybdenum 1781 Smelt with carbon Molybdenite MoS2
Tungsten 1783 Smelt with carbon Wolframite (Fe,Mn)WO4
Chromium 1797 Smelt with carbon Crocoite PbCrO4
Palladium 1802 Isolated from Pt
Osmium 1803 Isolated from Pt
Iridium 1803 Isolated from Pt
Rhodium 1804 Isolated from Pt
Sodium 1807 Electrolysis
Potassium 1807 Electrolysis
Magnesium 1808 Electrolysis Magnesia MgCO3
Cadmium 1817 Isolated from zinc
Lithium 1821 Electrolysis of LiO2 Petalite LiAlSi4O10
Zirconium 1824 Smelt with potassium Zircon ZrSiO4
Aluminum 1827 Smelt with potassium
Silicon 1823 Smelt with potassium
Beryllium 1828 Smelt with potassium Beryl Be3Al2Si6O18
Thorium 1929 Smelt with potassium Gadolinite (Ce,La,Nd,Y)2FeBe2Si2O10
Vanadium 1831 Smelt VCl2 with H2 Vanadinite Pb5(VO4)3Cl
Uranium 1841 Smelt with potassium Uranite UO2
Ruthenium 1844 Isolated from Pt
Tantalum 1864 Smelt with hydrogen Tantalite [(Fe,Mn)Ta2O6]
Niobium 1864 Smelt with hydrogen Tantalite [(Fe,Mn)Ta2O6]
Fluorine 1886 Electrolysis
Helium 1895 From uranium ore
Titanium 1910 Smelt with sodium Ilmenite FeTiO3
Hafnium 1924 Isolated from zirconium
Rhenium 1928 Isolated from Pt
Scandium 1937 Electrolysis Gadolinite FeTiO3
-384 -322 Aristotle. Wrote "Meteorology"
-370 -285 Theophrastus. Wrote "De Mineralibus"
77 Pliny the Elder publishes "Natural History"
973 1050 Al Biruni. Published "Gems"
1546 Georgius Agricola publishes "On the Nature of Rocks"
1556 Georgius Agricola publishes "On Metals"
1609 de Boodt publishes a catalog of minerals
1669 Brand: Discovery of phosphorus
1714 John Woodward publishes "Naturalis historia telluris illustrata & aucta", a mineral catalog
1735 Brandt: Discovery of cobalt
1777 Lavoisier: Discovery of sulfur
1778 Lavoisier: Discovery of oxygen and prediction of silicon
1783 Lavoisier: Discovery of hydrogen
1784 T. Olof Bergman publishes "Manuel du mineralogiste, ou sciagraphie du regne mineral",
and founds analytical chemistry
1778 Lavoisier: Discovery of oxygen
1801 Rene Just Huay publishes "Traite de Mineralogie", founding crystallography
1811 Avogadro publishes "Avogadro's law"
1860 The Karlsruhe Congress publishes a table of atomic weights
1869 Mendeleev publishes the periodic table
Shear modulus = S = 82 GJoules/meter3
Density = D = 7900 kg/meter3
Sword worthiness = Q = S/D = 10.4 MJoules/kg


-600 Wootz steel developed in India and is renowned as the finest steel in the world.
1700 The technique for making Wootz steel is lost.
1790 Wootz steel begins to be studied by the British Royal Society.
1838 Anosov replicates Wootz steel.
Wootz steel is a mix of two phases: martensite (crystalline iron with .5% carbon),
and cementite (iron carbide, Fe, 6.7% carbon).





Cu2O + C → 2 Cu + CO
At low temperature copper stays in the form of Cu2O and at high
temperature it gives the oxygen to carbon and becomes pure copper.
3 Fe2O3 + C → 2 Fe3O4 + CO
Fe3O4 + C → 3 FeO + CO
FeO + C → Fe + CO
Oxidation state = Number of electrons each iron atom gives to oxygen
Oxidation state
CuO 2
Cu2O 1
Cu 0
Fe2O3 3
Fe3O4 8/3
FeO 2
Fe 0
Smelt Method Year Abundance
(C) (ppm)
Gold <0 * Ancient .0031
Silver <0 * Ancient .08
Platinum <0 * 1735 .0037
Mercury <0 heat -2000 .067
Palladium <0 chem 1802 .0063
Copper 80 C -5000 68
Sulfur 200 * Ancient 420
Lead 350 C -6500 10
Nickel 500 C 1751 90
Cadmium 500 C 1817 .15
Cobalt 525 ? 1735 30
Tin 725 C -3200 2.2
Iron 750 C -1000 63000
Phosphorus 750 heat 1669 10000
Tungsten 850 C 1783 1100
Potassium 850 e- 1807 15000
Zinc 975 C 1746 79
Sodium 1000 e- 1807 23000
Chromium 1250 C 1797 140
Niobium 1300 H 1864 17
Manganese 1450 C 1774 1120
Vanadium 1550 ? 1831 190
Silicon 1575 K 1823 270000
Titanium 1650 Na 1910 66000
Magnesium 1875 e- 1808 29000
Lithium 1900 e- 1821 17
Aluminum 2000 K 1827 82000
Uranium 2000 K 1841 1.8
Beryllium 2350 K 1828 1.9
Smelt: Temperature required to smelt with carbon
Method: Method used to purify the metal when it was first discovered
*: The element occurs in its pure form naturally
C: Smelt with carbon
K: Smelt with potassium
Na: Smelt with sodium
H: Smelt with hydrogen
e-: Electrolysis
heat: Heat causes the oxide to decompose into pure metal. No carbon required.
chem: Chemical separation
Discovery: Year the element was first obtained in pure form
Abundance: Abundance in the Earth's crust in parts per million
Elements with a low carbon smelting temperature were discovered in ancient
times unless the element was rare. Cobalt was discovered in 1735, the first new
metal since antiquity, and this inspired scientists to smelt every known
mineral in the hope that it would yield a new metal. By 1800 all the rare
elements that were carbon smeltable were discovered.

Fe2O3 + 2 Al → 2 Fe + Al2O3
Oxidation state Oxidation state
at left at right
2 M2O ↔ 4 M + O2 1 0
4 MO ↔ 2 M2O + O2 2 1
2 M3O4 ↔ 6 MO + O2 8/3 2
6 M2O3 ↔ 4 M3O4 + O2 3 8/3
2 M2O3 ↔ 4 MO + O2 3 2
2 MO ↔ 2 M + O2 2 0
2/3 M2O3 ↔ 4/3 M + O2 3 0
1 MO2 ↔ 1 M + O2 4 0
2 MO2 ↔ 2 MO + O2 4 2
.jpg)













2(OH)6.jpg)













_.jpg)







.jpg)






.jpg)






_3.jpg)












-178918.jpg)










© Jason Maron, all rights reserved.