



 |
 |
 |
 |
|---|---|---|---|
The fundamental units are the meter, second, kilogram, and Coulomb. They were defined in 1793 as the "Standard International" (SI) units, or "MKS" units.
Quantity Unit Definition
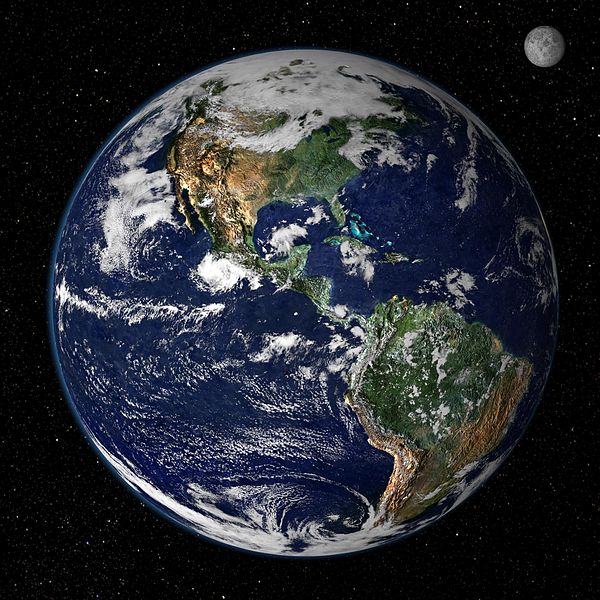
Length Meter The Earth's circumference is 40 million meters
Time Second There are 86400 seconds in one Earth day
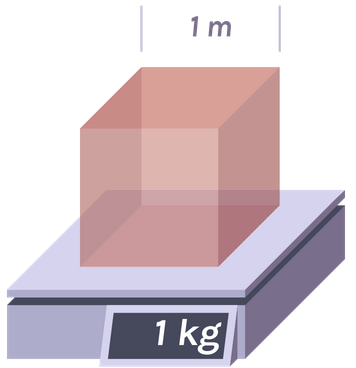
Mass Kilogram The mass of a cube of water 10 cm on a side is 1 kilogram
Charge Coulomb The force between two charges of one Coulomb each and
separated by 1 meter is 9 billion Newtons
 |
 |
 |
|---|---|---|
Density of water =1000 kg/meter = 1 g/cm Density of air = 1.2 kg/meter = .0012 g/cm
The fundamental units are length, mass, time, and charge, and all other units are derived from these.
Quanity Unit Composition Length meter X meter Mass kg M kg Time second T second Charge Coulomb C Coulomb Speed V = X/T Length / Time meter/second Acceleration A = V/T Speed / Time meter/second2 Momentum Q = M V Mass * Speed kg meter/second Force Newtons F = M A Mass * Acceleration kg meter/second2 Energy Joule E = F X Force * Distance kg meters2/second2 Power Watt P = E/T Energy / Time kg meters2/second3 Area S = X2 Length meters Volume Υ = X3 Length meters Density ρ = M/Υ Mass / Volume kg / meters2 Pressure Pascal Φ = F/S Force / Area Newtons/meter = Joules/meter Angular momentum L = M V X Momentum*Length kg meters/second Torque Γ = F X Force * Length kg meters/second Frequency Hertz f = 1/T 1 / Time 1/second
Meter = 3.281 feet
= 39.37 inches
Mile = 5280 feet (exact)
= 1609 meters
Foot = 12 inches (exact)
Inch = 25.4 mm (exact)
Minute = 60 seconds (exact)
Hour = 60 minutes (exact)
Day = 24 hours (exact)
Year = 365.25 days
Ton = 1000 kg (exact)
Kilogram = 1000 grams (exact)
= 2.205 pounds (pounds interpreted as mass)
Newton = .225 pounds (pounds interpreted as force)
Pound = 16 ounces (exact) (interpreted as mass)
= .454 kg
4.448 Newtons (Newtons interpreted as force)
Ounce = 28.35 grams (ounces interpreted as mass)
Meter/second = 2.24 miles/hour
Km/hour = .621 miles/hour
Miles/hour = 1.609 km/hour
Pascal = .0001450 pounds/inch2 (pounds interpreted as force)
Pound/inch2 = 6895 Pascals
Bar =101325 Pascals (Atmosphere pressure at sea level)
= 14.50 pounds/inch2 (pounds interpreted as force)
Earth gravity= 9.807 meters/second2
= 32.2 feet/second2
Standard sheet of paper = 11 x 8.5 inches = 27.94 x 21.59 cm
Speed of light 2.9979e8 m/s
Gravitational constant 6.6738e-11 m3/kg/s2
Planck constant 6.6261e-34 J s
Earth surface gravity 9.8067 m/s
Electric force constant 8.9876e9 N m2 / C2
Magnetic constant 4 Pi e-7 N/A2
Proton mass 1.6726e-27 kg = 938.272 GeV
Neutron mass 1.6749e-27 kg = 939.565 GeV
Electron mass 9.1094e-31 kg
Electron charge 1.6022e-19 C
Atomic mass unit 1.6605e-27 kg
Bohr radius 5.2918e-11 m = hbar2 / (ElectronMass*ElectronCharge2*Ke)
Boltzmann constant 1.3806e-23 J/K
Avogadro number 6.0221e23 particles/mole
Gas constant 8.3145 J/K/mole
Stefan-Boltzmann constant 5.6704e-8 Watts/m2/K4
Wein constant 2.8978e-3 m K
Mole of Carbon-12 .012 kg Exact
Planck length 1.6162e-35 m
Planck mass 2.1765e-8 kg
Planck time 5.3911e-44 s
Planck charge 1.8755e-18 C
Planck temperature 1.4168e32 K
Water heat capacity 4200 J/kg/K
Steam heat capacity 2080 J/kg/K At 100 Celsius
Ice heat capacity 2110 J/kg/K At -10 Celsius
Air heat capacity 1004 J/kg/K
Stefan-Boltzmann 5.67e-8 Watts/meter2/Kelvin4
= (2π5/15) Boltzmann4 / SpeedOfLight2 / PlanckConstant3
Wein 2.898e-3 Kelvin meters
Electron spin 5.2729e-35 Joule seconds = PlanckConstant / (4 Pi)
Pi 3.14159
Euler number 2.71828
System Units Best suited for
SI (MKS) Meters, Kilograms, Seconds Newtonian mechanics, EM forces between currents
Gaussian (CGS) Centimeters, Grams, Seconds EM forces between particles, plasma physics, astrophysics
Particle Meters, Electron Volts, Seconds Particle physics
Planck Planck length, Planck mass, Planck time General relativity, quantum gravity
1 gram = .001 kg
1 cm = .01 meters
1 electron Volt (eV) = 1.602e-19 Joules
= The energy gained by an electron upon descending a potential of 1 Volt
Meters Earth Earth Light travel
radii orbits time
(AU)
Nucleus 2⋅10
Atom 2⋅10
Green photon 5.5⋅10
Neuron .00002
Dime thickness .00135
Dime diameter .0178
Quarter diameter .024
Tennis ball diameter .067
Soccer ball diameter .22
Average person 1.78
Central Park width 800
Mount Everest 8848
Moon radius 1737000 .273
Mars radius 3390000 .532
Earth radius 6371000 1.0
Jupiter radius 6.991⋅107 10.9
Moon distance 3.844⋅108 60.3 .00257 1.5 seconds
Sun radius 6.957⋅108 109 .00474 2.3 seconds
Earth orbit 1.496⋅1011 23481 1.0 8 minutes
Jupiter orbit 5.2 40 minutes
Neptune orbit 30.1 3 days
Light year 9.461⋅1015 63241 1 year
Alpha Centauri 4.4 years Nearest star
Galaxy thickness 1000 years
Galaxy center 27200 years
Galaxy diameter 100000 years
Andromeda distance 2.5 million years
Virgo cluster distance 54 million years
Size of universe 14 billion years
 |
 |
 |
 |
 |
|
|---|---|---|---|---|---|
 |
|---|
 |
|---|
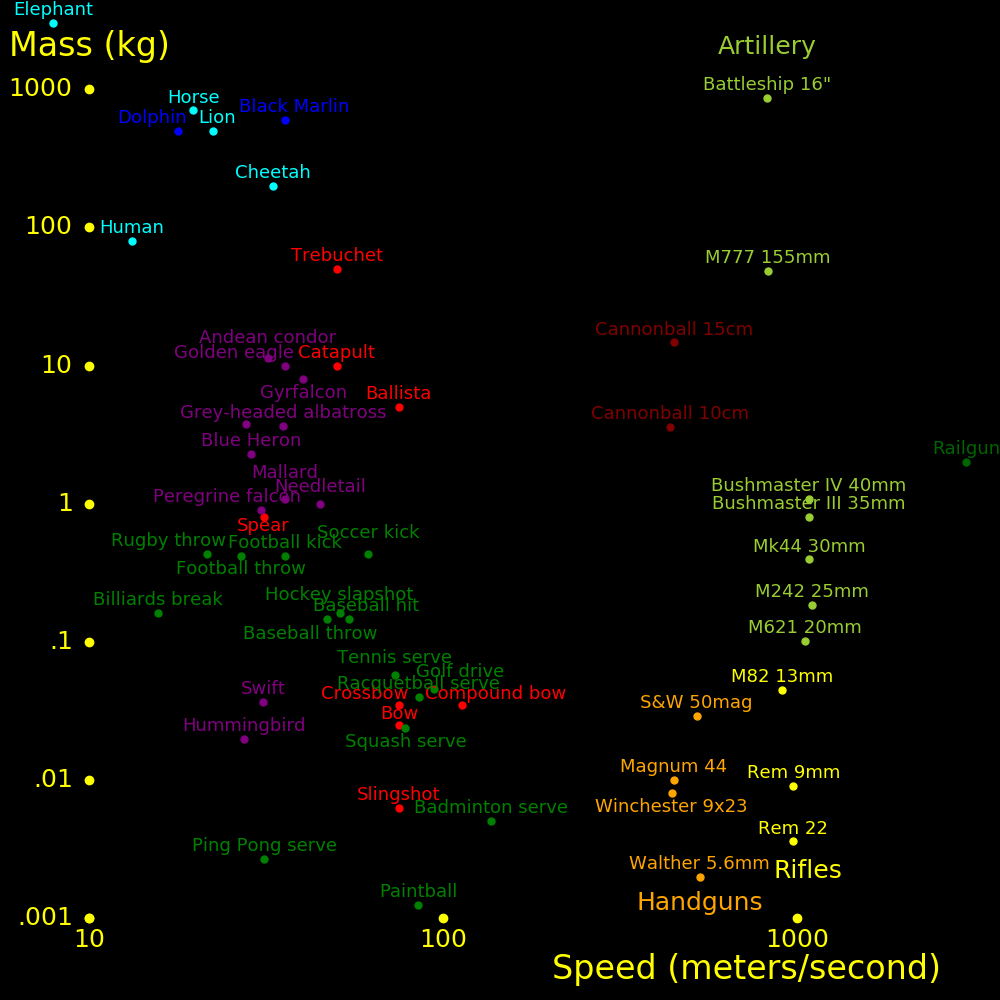
meters/second Mach
Walk 1.5
Running sprint 10
Cycling sprint 20
Cheetah 30 Fastest land animal
70 miles/hour 31
Baseball pitch 45 100 miles/hour
Human neuron 100
Sound at altitude 295 Speed of sound at altitude 10 km to 20 km
747 airplane 266 .9
Sound at sea level 340 1.0 At sea level and 15 degrees Celsius
F-35 Lightning 475 1.6 Stealth fighter
F-16 Falcon 590 2.0
Concorde 606 2.05
F-22 Raptor 670 2.3 Stealth fighter
F-15 Eagle 740 2.5
SR-71 Blackbird 980 3.3
Orbit speed 7800 26.4 Minimum speed to orbit the Earth
Escape speed 11200 38.0 Minimum speed to escape the Earth's gravity
Ion rocket 100000 Fastest spacecraft we can build
Fission rocket 107
Fusion rocket 107
Light 3⋅108 1020000
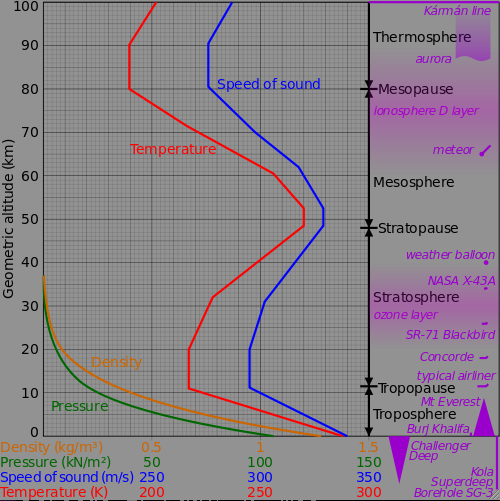
Aircraft typical fly at altitude 10 km to 20 km, where the speed of sound is
295 m/s. Mach 1 for aircraft is defined using this speed.
 |
 |
 |
|---|---|---|
kg Earth Solar
masses masses
Electron 9.109⋅10-31
Proton 1.673⋅10-27
Neutron 1.675⋅10-27
1 ounce .0283
Tennis ball .058
Soccer ball .44
1 pound .454
Typical human 70
Sumo wrestler 200
Ton 1000
Honda Civic 1200
Elephant 5000
Bradley tank 27000
Argentinosaurus 70000 Largest dinosaur
Blue whale 200000
Moon 7.35⋅1022 .0123
Mars 6.42⋅1023 .107
Earth 5.92⋅1024 1
Jupiter 1.90⋅1027 318 .00096
Sun 1.99⋅1030 330000 1.0
White dwarf max 2.9⋅1030 1.44
Milky Way black hole7.4⋅1036 4.2 million
Milky Way 2.5⋅1042 1.2 trillion
Andromeda 2.5⋅1042 1.2 trillion
M87 galaxy 10 trillion
Virgo galaxy cluster 1200 trillion
 |
|---|
 |
|---|
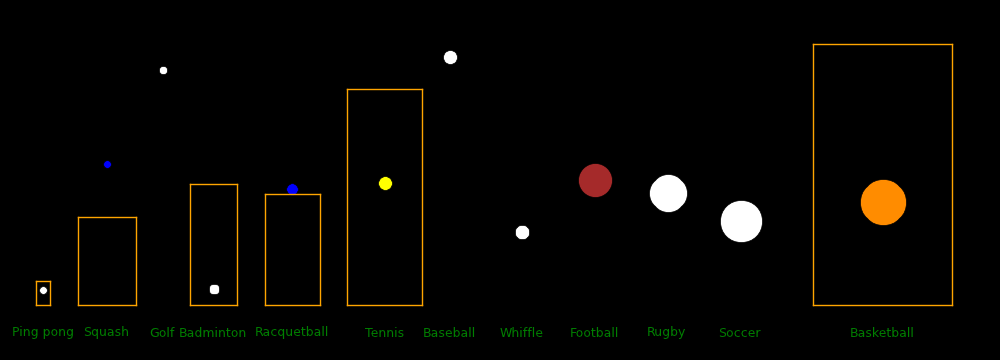
Ball Ball Court Court Ball Racquet Racquet Racquet Fastest Max Drag
diameter Mass length width density mass max length max width shot distance distance
mm gram meter meter gram/cm3 gram cm cm m/s meter meter
Ping pong 40 2.7 2.74 1.525 .081 70 31.2 1.8
Squash 40 24 9.75 6.4 .716 78.22 15.6
Golf 43 46 1.10 94.3 214.2 25.9
Paintball 1.25 85
Snooker 52.5 149 3.658 1.829
Badminton 54 5.1 13.4 5.18 .062 85 136.9 1.8
Racquetball 57 40 12.22 6.10 .413 85.4 12.8
Billiards 59 163 2.84 1.42 1.52 15.6 48.7
Tennis 67 58 23.77 8.23 .368 73.2 13.4
Cricket 72 160 80 .82 128.6 32.8 Throw 80ii meters from batter to home run boundary
158 Hit
Field hockey 73 160 91.4 55 .78
Baseball 74.5 146 122 .675 46.9 135.88 27.3 Throw Pitcher-batter distance = 19.4 m
54.14 177 Hit
Pickleball 74 24 13.41 6.10 .151 61.0 21.0
Hockey puck 76 163 61 26 1.44 51.0 25 mm thick
Whiffle 76 45 .196 8.1
Polo 82 130 274.3 146.3 .45
Croquet 92 454 1.11
Softball 97.1 188 .39 97.8 Throw
Softball 175.56 Hit
Football 178 420 91.44 48.76 .142 26.8 69.5 13.8 Throw
35.8 ~65 Placekick
90.5 Punt
Rhythmic gymn 190 400 12 12 .111
Rugby 191 435 100 70 .119 21.46 12.4 Throw
Volleyball 210 270 18 9 .056
Bowling 217 7260 18.29 1.05 1.36 160
Soccer 220 432 105 68 .078 35.84 9.3 Placekick
75.35 Punt
59.82 Throw-in
61.26 Throw
Basketball 239 624 28 15 .087 11.4
Disc ultimate 273 64 37 27.3 mm thick 18 meter end zonesDisc golf 300 200
Beach ball 610 120 .0011
Javelin 800 270 98.48
Discus 219 2000 74.08 44 mm thick
Hammer 102 7260 121.3 86.74
Shot put 125 7260 7.10 23.56
Cannonball 220 14000 7.9 945
Sumo 4.55
"Fastest shots" are world records.
 |
|---|
grams/cm2 $/kg Year of discovery
Magnesium 1.74 2.8 1808
Aluminum 2.70 1.7 1827
Titanium 4.51 10 1910
Zinc 7.14 2.0 1300
Manganese 7.21 2.3 1774
Iron 7.9 .3 -1200
Nickel 8.91 15 1751
Copper 8.96 6 -5000
Silver 10.49 640 Ancient
Lead 11.3 2 -6500
Tungsten 19.25 50 1783
Gold 19.30 43000 Ancient
Platinum 21.45 37000 1735
Osmium 22.59 12000 1803 Densest element
Air at Everest .0004 10 km altitude
Air at Denver .001 1 Mile altitude
Air at sea level .00127
Ice .92
Water 1.0
Rock 2.8
Earth 5.52
Moon 3.35
Mars 3.95
Europa 3.10
Ganymede 1.94
Callisto 1.83
Titan 1.88
Balsa .12
Corkwood .21
Cedar .32
Pine .37
Spruce, red .41
Oak, red .66
Hickory .81
Bamboo .85
Oak, live .98
Ironwood 1.1
Lignum Vitae 1.26
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
|---|
Mass Diameter Height Density Price/kg Copper Nickel Zinc Manganese
g mm mm g/cm3 $/kg fraction fraction fraction fraction
Penny 2.5 19.05 1.52 5.77 4.0 .025 .975
Nickel 5.000 21.21 1.95 7.26 10.0 .75 .25
Dime 2.268 17.91 1.35 4.62 44.1 .9167 .0833
Quarter 5.670 24.26 1.75 6.29 44.1 .9167 .0833
Half dollar 11.340 30.61 2.15 7.90 44.1 .9167 .0833
Dollar 8.100 26.5 2.00 7.53 123.5 .885 .02 .06 .035
Dollar bill 1.0 .11 .88 1000
The above objects are all to scale. The dimensions of a dollar bill are
155.956 mm * 66.294 mm * .11 mm.
Mass = M Diameter = D Height = H Volume = Vol = π H D2 / 4 Density = M / VolGold was the densest element known until the discovery of tungsten in 1783 and was hence valuable as an uncounterfeitable currency. Silver can be counterfeited with lead because lead is more dense and cheaper than silver.
The price of the metal in a penny is
Metal price = Penny mass * (Copper fraction * Copper price/kg + Zinc fraction * Zinc price/kg)
= .0025 kg * ( .025 6 $/kg .975 2 $/kg )
= .0052 $
For a penny made of pure copper the price of the metal is 1.5 cents.
A penny made of gold, silver, or zinc has a value of:
Price/Mass Price
$/kg $
Zinc 2 .005
Copper 6 .015
Silver 640 1.6
Gold 43000 108
 |
|---|
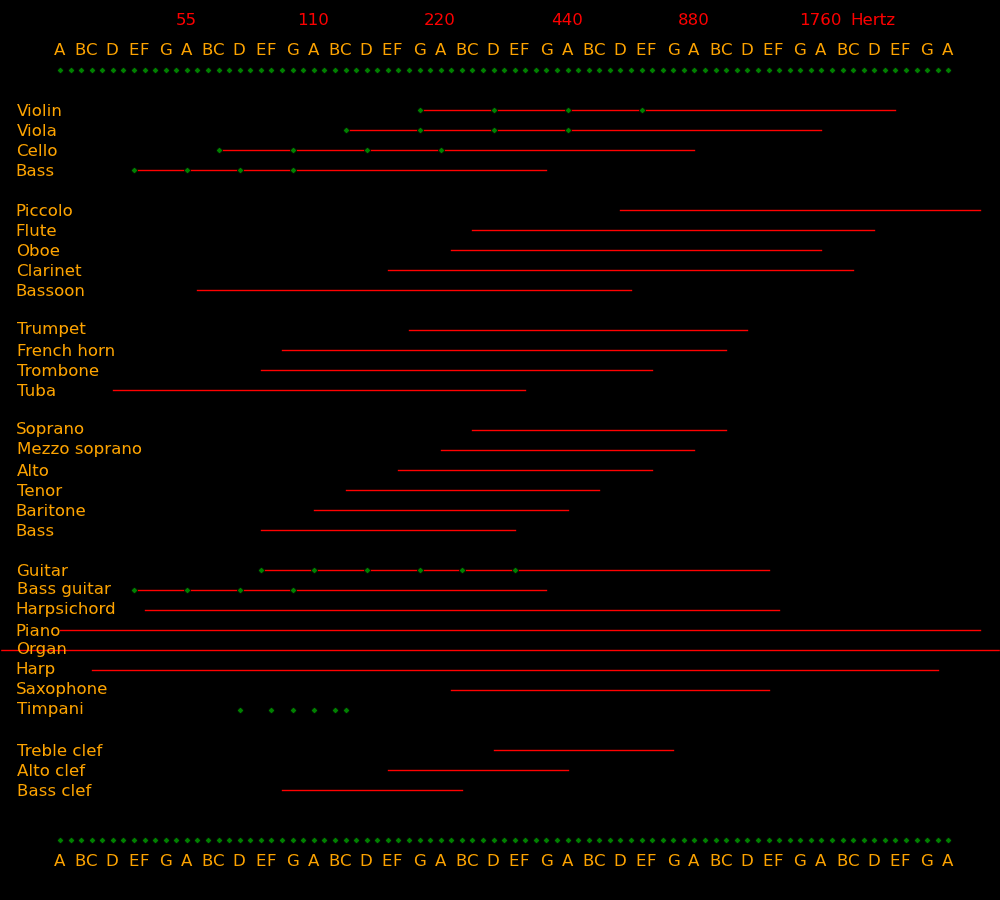
Frequency Note
(Hertz)
Whale songs 10
Human ear lower limit 20
Bass lowest note 41 E
Bass guitar lowest note 41 E
Cello lowest note 65 C
Bass singer lowest note 82 E
Viola & tenor lowest note 131 C
Violin & alto lowest note 196 G
Soprano lowest note 262 C
Violin D string 293 D
Violin A string 440 A
Violin E string 660 E
Human ear upper limit 20000
 |
 |
 |
 |
|---|---|---|---|
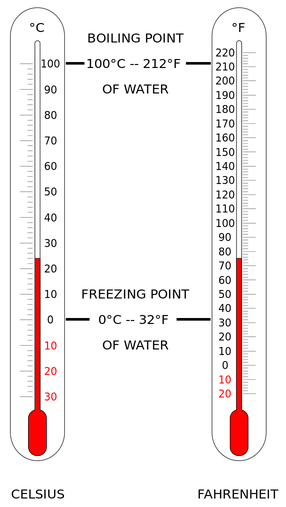
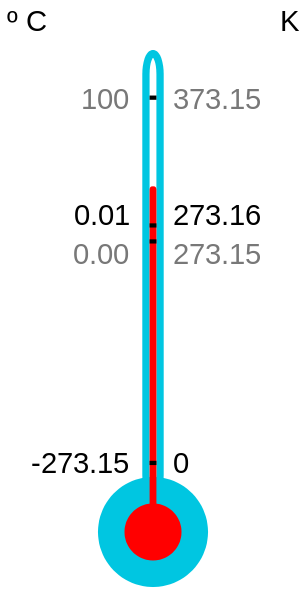
Kelvin Celsius Fahrenheit
Absolute zero 0 -273.2 -459.7
Water melting point 273.2 0 32
Room temperature 294 21 70
Human body temperature 310 37 98.6
Water boiling point 373.2 100 212
Kelvin
Absolute zero 0
Helium boiling point 4.2
Hydrogen boiling point 20.3
Triton 38
Pluto 44
Titania 70
Nitrogen boiling point 77.4
Oxygen boiling point 90.2
Titan 94
Europa 102
Hottest superconductor 135 HgBaCaCuO
Ceres 168
Mars 210
Water melting point 273.15
Earth average 288
Room temperature 293
Water boiling point 373.15
Venus 740
Wood fire 1170
Copper melting point 1358
Iron melting point 1811
Bunsen burner 1830
Tungsten melting point 3683 Highest melting point among metals
Earth's core 5650 Inner-core boundary
Sun's surface 5780
Solar core 13.6 million
Helium-4 fusion 200 million
Carbon-12 fusion 230 million
Color of a blackbody as a function of temperature (in Kelvin).
 |
|---|
Surface area = A Force = F Pressure = P = F / A (Pascals or Newtons/meter2 or Joules/meter3)
 |
|---|
Mass of the Earth's atmosphere = M = 5.15e18 kg
Surface area of the Earth = A = 5.10e14 m2
Gravitational constant = g = 9.8 m/s2
Pressure on Earth's surface = P = M g / A = 101000 Pascals
= 15 pounds/inch2
= 1 Bar
One bar is defined as the Earth's mean atmospheric pressure at sea level
Height Pressure Density
(km) (Bar) (kg/m3)
Sea level 0 1.00 1.225
Denver 1.6 .82 1.05 One mile
Everest 8.8 .31 .48
Airbus A380 13.1 .16 .26
F-22 Raptor 19.8 .056 .091
SR-71 Blackbird 25.9 .022 .034
Space station 400 .000009 .000016
Energies in Joules.
1 food calorie 4200 1 Watt hour 3600 1 Watt * 3600 seconds Sprinting person 2560 80 kg moving at 8 m/s Battery, lithium, CR1216 330 Smallest button cell Battery, lithium, CR2032 3000 Most common button cell Battery, lithium-ion, AAAA 2300 Battery, lithium-ion, AAA 4700 Battery, lithium-ion, AA 9000 Battery, lithium-ion, A 47000 Battery, lithium-ion, B 58000 Battery, lithium-ion, C 67000 Battery, lithium-ion, D 107000 Battery, iPhone 7 (5 inch) 40000 Battery, Samsung S6 (5 inch) 52000 Battery, iPad mini (8 inch) 59000 Battery, iPad Pro (10 inch) 100000 Battery, iPad Pro (13 inch) 148000
Energies in MJoules:
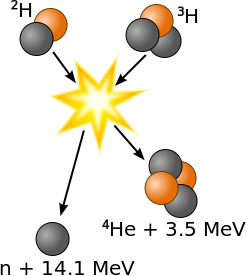
1 kg of Lithium-ion battery .80 1 kg of TNT 4.2 1 kg of sugar 20 = 5000 Food Calories 1 kg of protein 20 = 5000 Food Calories 1 kg of alcohol 25 = 7000 Food Calories 1 kg of fat 38 = 9000 Food Calories 1 kg of gasoline 48 = 13000 Food Calories Tesla Model 3 battery 270 Fission bomb, uranium 8⋅107 = 20 kilotons of TNT Fusion bomb 8⋅1010 = 20 megatons of TNT World energy used in 1 year 6⋅1014
Forms of energy:
Distance = X meters Force = F Newtons Mass = M kg Velocity = V meters/second Gravity constant = g = 9.8 meters/second2 Pressure = P Pascals Volume = U meters3 Mechanical energy= Ew = F X Joules Gravity energy = Eg = MgX Joules (X = height above ground) Kinetic energy = Ek = ½MV2 Joules Pressure energy = Ep = P U Joules
 |
|---|
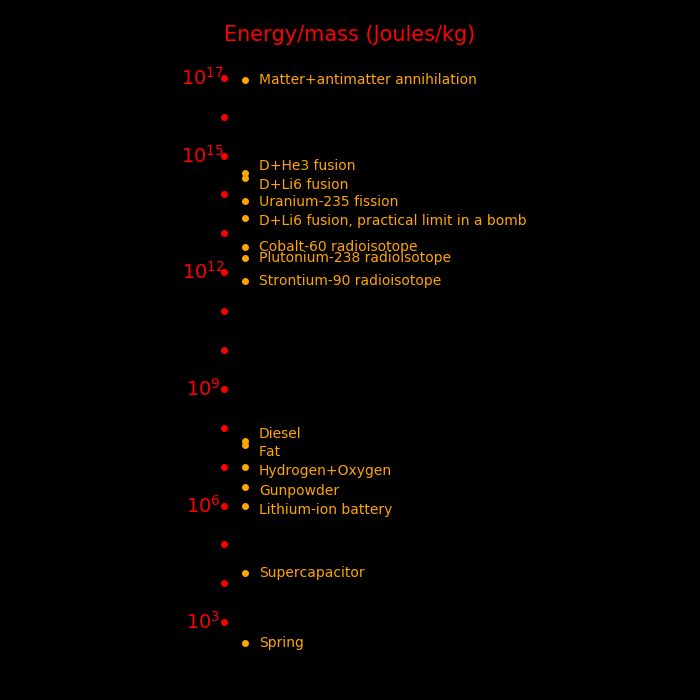
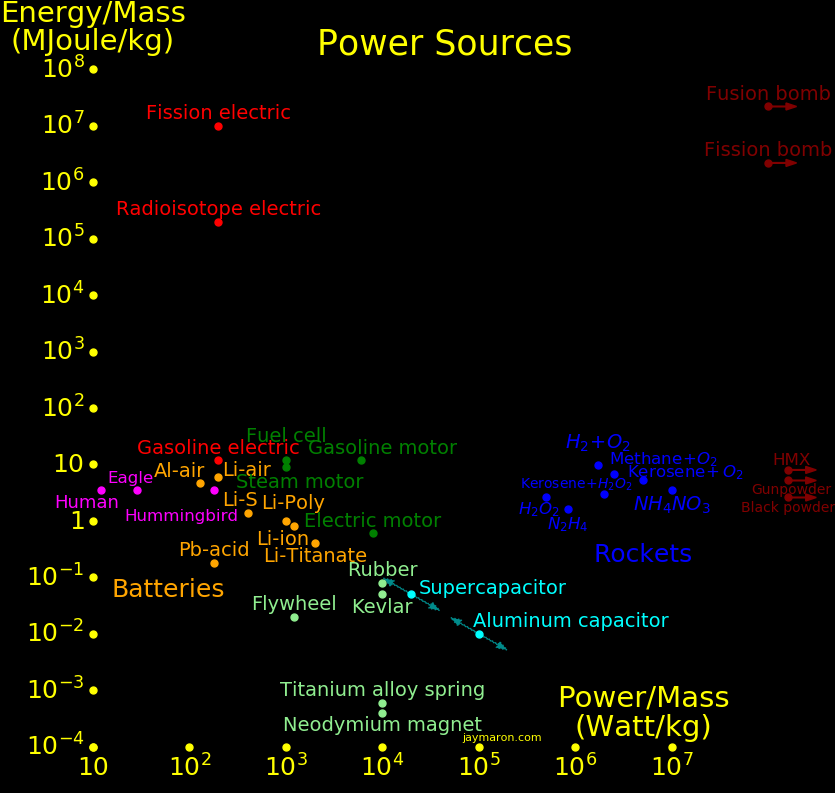
Energy/Mass Mass fraction
MJoule/kg
Antimatter 90,000,000,000 1
Fusion, D + Li6 268,000,000 .00298
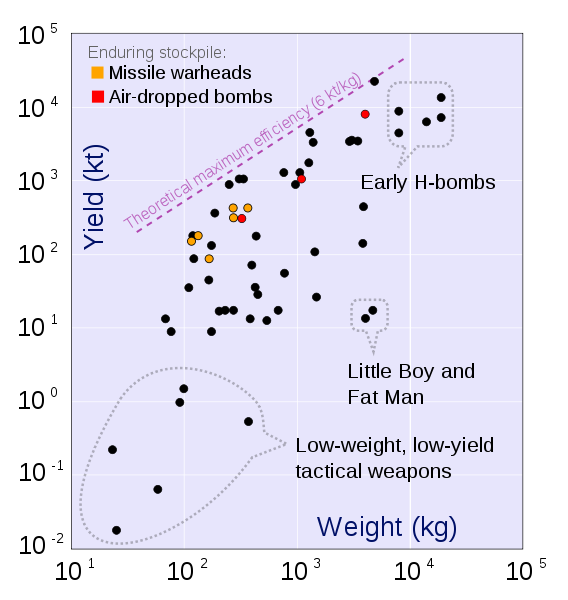
Fusion bomb 25,000,000 .000278 Maximum practical yield of a bomb
Fission, U-235 83,000,000 .000918
Fission bomb 6,000,000 .000067 Maximum practical yield of a bomb
Fission, fast neutron 28,000,000 Fast neutrons, unenriched fuel
Fission, slow neutron 500,000 Slow neutrons, unenriched fuel
Nuclear battery, Co60 4,300,000 Half life 5.3 year
Nuclear battery, Pu238 2,260,000 Half life 88 year
Nuclear battery, Pu241 1,960,000 Half life 14.4 year
Nuclear battery, Sr90 590,000 Half life 29 year
Hydrogen 141.8
Methane 55.5 1 carbon. Natural gas
Ethane 51.9 2 carbons
Propane 50.4 3 carbons
Butane 49.5 4 carbons
Octane 47.8 8 carbons
Kerosene 46 12 carbons
Diesel 46 16 carbons
Oil 46 36 carbons
Fat 37 20 carbons. 9 Calories/gram
Pure carbon 32.8
Coal 32 Similar to pure carbon
Ethanol 29 7 Calories/gram
Wood 22
Sugar 17 4 Calories/gram
Protein 17 4 Calories/gram
Plastic explosive 8.0 HMX
Smokeless powder 5.2 Modern gunpowder
TNT 4.7
Black powder 2.6 Medieval gunpowder
Phosphocreatine .137 Recharges ATP
ATP .057 Adenosine triphosphate
Aluminum capacitor .010
Spring .0003
Battery, aluminum-air 4.68
Battery, Li-S 1.44
Battery, Li-ion .8
Battery, Li-polymer .6
Battery, Alkaline .4
Battery, Lead acid .15
"Mass fraction" is the fraction of mass converted to energy, by E=MC2.
 |
|---|
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
 |
 |
|---|---|---|
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
 |
 |
 |
 |
|---|---|---|---|---|
 |
 |
|---|---|
 |
 |
 |
 |
 |
 |
 |
|
|---|---|---|---|---|---|---|---|
Energy = E Joules Time = T seconds Power = P = E/T Watts Mass = M kilograms Energy/Mass = e = E/M Joules/kilogram Power/Mass = p = P/M Watts/kilogram
Watts
Human cell 10-12
iPhone 7, standby .05
iPhone 7, audio .3
iPhone 7, video .9
iPhone 7, talk .9
iPad Pro 10 inch, idle 3
Human brain 20
Incandescent Light bulb 60
Human at rest 100
Unstrenuous cycling 200
1 horsepower 746
Strenuous cycling 600
Maximum human power 1600
World power per person 2500
Tesla S Ludicrous 397000 532 horsepower
Wind turbine 1⋅106
Blue whale 2.5⋅106
Boeing 747 1.4⋅108
Hoover Dam 2.1⋅109
U.S. power consumption 3.4⋅1012
World power consumption 1.5⋅1013
Earth geologic heat 4.4⋅1013
World photosynthesis 7.5⋅1013
Earth solar power 1.7⋅1017 Total solar power falling on the Earth
 |
|---|
Energy/Mass Power/Mass
MJoule/kg Watts/kg
Battery, Lithium ion .8 1200
Battery, Lithium polymer 1.0 1000
Battery, Lithium titanate .4 4000
Battery, Lithium sulfur 1.8 800
Battery, Lithium air 6.1 200
Battery, Aluminum air 4.6 130 Not rechargeable
Capacitor, Aluminum, high power .01 100000
Capacitor, Aluminum, high energy .1 10000
Gasoline combustion motor - 8000
Electric motor - 8000
Electric generator - 200
Flywheel .02 200
Rocket, H2O2 2.7 1000000
Rocket, NH3 + H2O2 6
Rocket, Kerosene + H2O2 8.1 2000000
Rocket, Methane + Oxygen 11.1 2500000
Rocket, Kerosene + Oxygen 10.3 5000000
Rocket, Hydrogen + Oxygen 13.2 1700000
Rocket, Al+NH4NO3 (solid fuel) 6.9 9000000
Nuclear alpha, Plutonium-241 200000 40
Nuclear beta, Tungsten-185 20000 40
Nuclear fission electric 10000000 40
Human, cycling sprint .000188 17.4
Eagle 42
Hummingbird 300
Fusion bomb, D + Li-6 22000000 Large
For gasoline, we assume conversion to electricity by a generator with an efficiency of 1/4.
Cycling measurements are from Menaspa's (2013) analysis of Tour de France sprints. For a 1020 Watt sprint the speed is 18.4 meters/second.
Meters/second2
Ceres gravity .27
Europa gravity 1.31
Titan gravity 1.35
Moon gravity 1.62
Mars gravity 3.8
Venus gravity 8.87
Earth gravity 9.8
Bugatti Veyron 15.2 0 to 100 km/h in 2.4 seconds
Red out 30 Max long-term acceleration in the direction of blood rushing to your head
Blackout 50 Max long-term acceleration while sitting
Formula-1 car 50 High-speed breaking and cornering with a downforce wing
Blackout with g suit 90 Max long-term acceleration while sitting with a g-suit
Max long-term (front) 120 Max long-term acceleration while lying on one's front
Max long-term (back) 170 Max long-term acceleration while lying on one's back
Max short-term 500 Max short-term acceleration
Bullet 310000 9x19 Parabellum handgun, average acceleration along the barrel
English units have the virtue of using base 2. The ideal base for mathematics is 16, because it is instantly interconvertible with base 2 and 4, and easily with base 8. Base 10 is a debacle because of the awkward prime factor "5" and the aliens are mocking us for it.
The volume units are:
Tablespoon= 4 drams = 3 teaspoons Ounce = 8 drams Jack = 2 ounces Gill = 4 ounces Cup = 8 ounces Pint = 16 ounces = 1 pound of water Quart = 2 pints Pottle = 4 pints Gallon = 8 pints Peck = 2 gallons Kenning = 4 gallons Bushel = 8 gallons Strike = 16 gallons Coomb = 32 gallons Seam = 64 gallons Barrel = 31.5 gallons Hogshead = 2 barrels Butt = 4 barrels Tun = 8 barrels = 2016 pounds of water = 914 kg = .914 metric tonsThese are equivalently units of mass, using water as the density conversion. 1 pint of volume is one pound of mass.
Inches are subdivided by powers of 2.
One box of paper has 10 reams, and each ream has 500 sheets.
Other units:
Quire = 25 sheets Ream = 20 quires = 500 sheets Bundle = 2 reams = 40 quires = 1000 sheets Bale = 10 reams = 200 quires = 5000 sheets = 1 box of paper digit = 3/4 inch finger = 7/8 inch palm = 3 inches hand = 4 inches span = 9 inches foot = 12 inches cubit = 18 inches yard = 36 inches ell = 45 inches fathom = 6 feet rod =16.5 feet Chain = 66 feet furlong = 660 feet mile =5280 feet = 320 rods knot =6086 feet league = 3 knot perch = 1 square rod square chain = 16 perches acre = 160 square rods = 1 chain X 1 furlong = 10 square chains square mile = 640 acres cord = 128 cubic feet (8x4x4) dram = 27.34 grains grain, unit = .06480 gram grain, barley = .065 gram grain, wheat = .050 gram grain, carob = .200 gram stone = 14 pounds (unit of mass) 1 pint = 1.0432 pounds of water 1 gallon = 8.345 pounds of water Short ton = 2000 pounds Long ton = 2240 pounds Metric ton = 2204.6 pounds 1 Tun volume of water = 2103 pounds drachma = 6 obols Greek drachma = 4.37 gram Roman drachma = 3.41 gram
Ideally, units should be based on Planck units, and the base should be 16.
An acre is the amount of land that can be plowed in one day by an ox.
 |
 |
|---|---|
 |
|---|
 |
|---|
In this plot, the diameter of each particle proportional to CubeRoot(Mass). This is what the particles would look like if they were uniform-density spheres.
The electron is exaggerated otherwise it would be invisible.
The blue particles represent the heaviest particle that can be produced by each accelerator.
At this scale, a Big Bang particle has a diameter of 10 km.
Photons, Gluons, and Gravitons are massless.
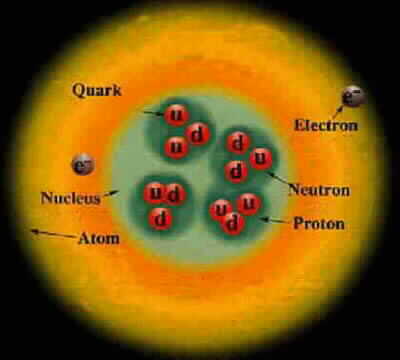
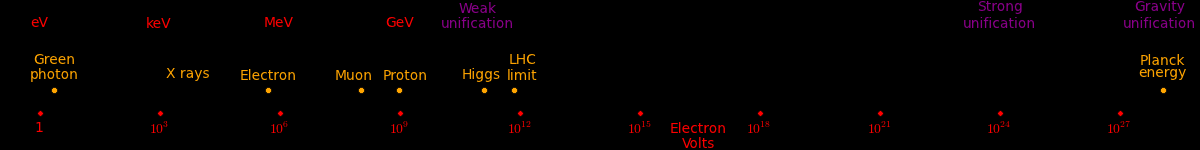
Electron neutrino < 1 eV Muon neutrino < 2 eV Red photon 1.8 eV Green photon 2.3 eV Blue photon 3.1 eV Electron .51 MeV Up quark 1.9 MeV Down quark 4.4 MeV Strange quark 87 MeV Muon 105.7 MeV Neutral pion 135 MeV Charged pion 140 MeV Proton 938.27 MeV Neutron 939.57 MeV Charm quark 1.32 GeV Discovered at SLAC Tau 1.78 GeV Discovered at SLAC Bottom quark 4.24 GeV Discovered at Fermilab SLAC limit 45 GeV Highest-energy particle that SLAC can produce W boson 80 GeV Discovered at the Super Proton Synchrotron Z boson 91 GeV Discovered at the Super Proton Synchrotron Fermilab limti 125 GeV Highest-energy particle that Fermilab can produce Higgs Boson 125 GeV Discovered at the LHC Top quark 173 GeV Discovered at Fermilab LHC limit 1000 GeV Highest-energy particle that the LHC can produce Cosmic rays 10^12 GeV Highest-energy events observed Planck energy 10^19 GeV Quantum gravity. Planck energy = 1.22e28 eV = 1.956e9 Joules 1 electron Volt (eV) = 1.602e-19 Joules ~ kT at 11,000 Kelvin
Quantity MKS units CGS units Conversion factor Mass M kg gram .001 Wire length Z meter cm .01 Radial distance from wire R meter cm .01 Time T second second 1 Force F Newton dyne 100000 Charge Q Coulomb Franklin 3.336e-10 Velocity of a charge V meter/second cm/s .01 Speed of light C 2.999e8 meter/second cm/s 100 Energy E Joule erg e-7 Electric current I Ampere = Coulomb/s Franklin/s 3.336e-10 Electric potential V Volt Statvolt 299.79 Electric field E Volt/meter StatVolt/cm 29979 Magnetic field B Tesla Gauss 10000 Capacitance C Farad cm 1.11e-12 Inductance L Henry s2/cm 9e-11 Electric force constant Ke = 8.988e9 N m2/C2 Ke = 1 dyne cm2 / Franklin2 Magnetic force constant Km = 2e-7 = Ke/C2 Km = 1/C2 Vacuum permittivity ε = 8.854e-12 F/m =1/4/π/Ke Vacuum permeability μ = 4 π e-7 Vs/A/m =2 π Km Proton charge Qpro = 1.602e-19 Coulomb Qpro= 4.803e-10 Franklin Electric field from a charge E = Ke Q / R2 E = Q / R2 Electric force on a charge F = Q E F = Q E Electric force between charges F = Ke Q Q / R2 F = Q Q / R2 Magnetic field of moving charge B = Km V Q / R2 B = (V/C) Q / R2 Magnetic field around a wire B = Km I / R B = (V/C) I / R Magnetic force on a charge F = Q V B F = (V/C) Q B Magnetic force on a wire F = Km B Z F = I B z Magnetic force between charges F = Km V2 Q1 Q2 / R2 F = (V/C)2 Q Q / R2 Magnetic force between wires F = Km I1 I2 Z / R F = I1 I2 Z / R Energy of a capacitor E = .5 C V2 Field energy per volume Z = (8 π Ke)-1 (E2 + B2/C2) Z = .5 (E2 + B2/C2)
Speed of light C Electric field E Electric field, time derivative Et Magnetic field B Magnetic field, time derivative Bt Charge Q Charge density q Current density J MKS CGS Ke=8.988e9 Ke=1 Km=2e-7 Km=2/C ∇˙E = 4 π Ke q ∇˙E = 4 π q ∇˙B = 0 ∇˙B = 0 ∇×E = -Bt ∇×E = -Bt / C ∇×B = 2 π Km J + Et / C2 ∇×B = 4 π J / C + Et / C
 |
|---|
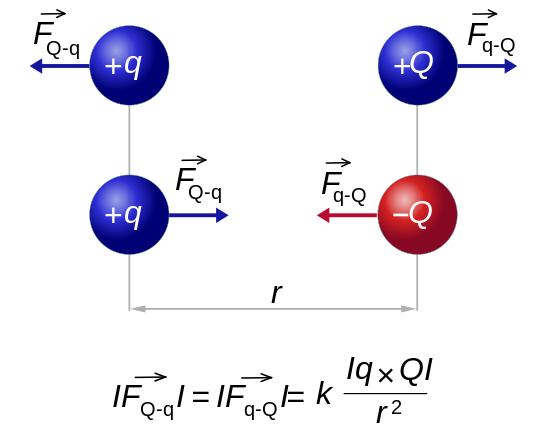
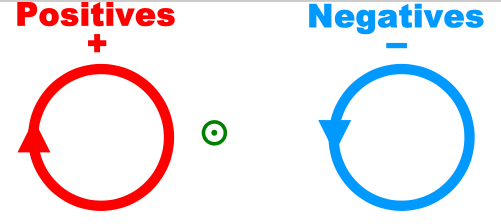
Charges of the same sign repel and charges of opposite sign attract.
Charge 1 Charge 2 Electric Force + + Repel - - Repel + - Attract - + Attract Charge = Q (Coulombs) 1 Proton = 1.602e-19 Coulombs Distance between charges = R Mass of the charges = M Gravity constant = G = 6.67e-11 Newton m2 / kg2 Electric constant = K = 8.99e9 Newton m2 / Coulomb2 Gravity force = F = -G M1 M2 / R2 = M2 g Electric force = F = -K Q1 Q2 / R2 = Q2 E Gravity field from M1 = g = G M1 / R2 Electric field from Q1 = E = K Q1 / R2 Gravity voltage = H g (H = Height, g = Gravitational acceleration) Electric voltage = H E (H = Distance parallel to the electric field) Gravity energy = -G M1 M2 / R Electric energy = -K Q1 Q2 / R
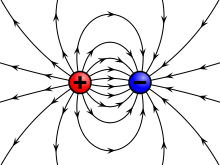 |
|---|
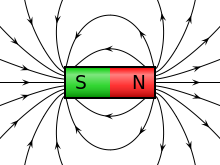
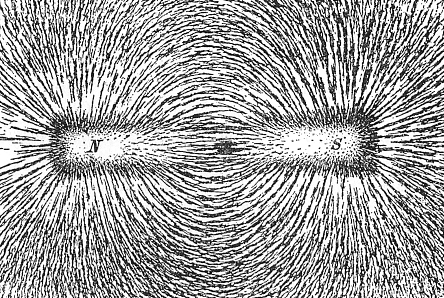
A charge generates an electric field. The electric field points away from positive charges and toward negative charges.
 |
 |
|---|---|
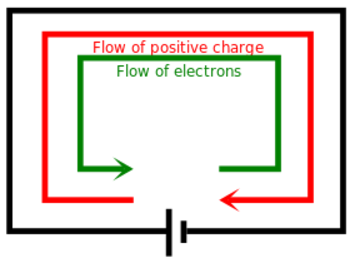
A moving charge is an "electric current". In an electric circuit, a battery moves electrons through a wire.
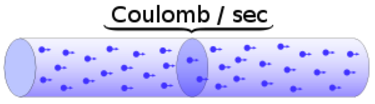
Charge = Q Time = T Electric current = I = Q / T (Coulombs/second)The current from a positive charge moving to the right is equivalent to that from a negative charge moving to the left.
 |
|---|
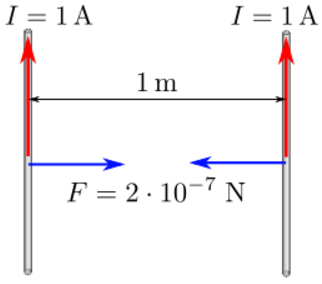
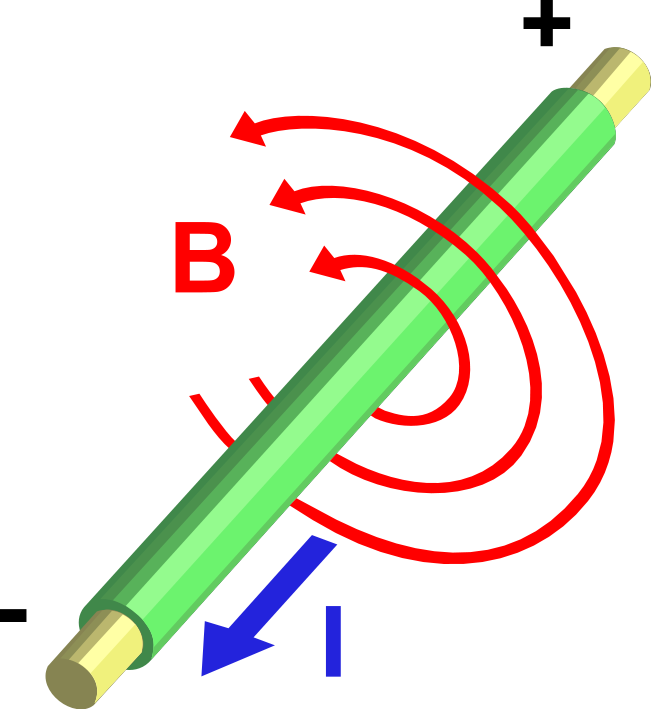
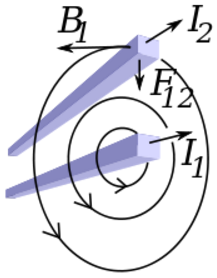
Moving charges and currents exert forces on each other. Parallel currents attract and antiparallel currents repel.
Charge = Q Velocity of the charges = V Current = I Length of a wire = L Distance between the charges = R Electric force constant = Ke = 8.988e9 N m2/C2 Magnetic force constant = Km = 2e-7 = Ke/C2 Electric force between charges = Fe = Ke Q1 Q2 / R2 Magnetic force between charges = Fm = Km V2 Q1 Q2 / R2 = (V2/C2) Fe Magnetic force between currents = Fm = Km I1 I2 Z / R Magnetic force / Electric force = V2 / C2The magnetic force is always less than the electric force.
 |
 |
|---|---|
The electric force can be interpreted as an electric field, and the magnetic force can be interpreted as a magnetic field. Both interpretations produce the same force.
Radial distance = R (Distance perpendicular to the velocity of the charge) Magnetic field from charge Q1 = B = Km V Q1 / R2 Magnetic field from current I1 = B = Km I1 / R Magnetic force on charge Q2 = Fm = Q2 V B = Km V2 Q1 Q2 / R2 Magnetic force on current I2 = Fm = I2 Z B = Km I1 I2 Z / R
 |
 |
|
|---|---|---|
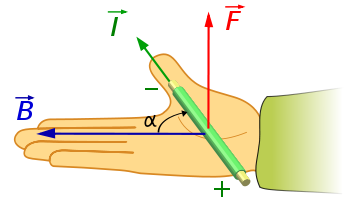
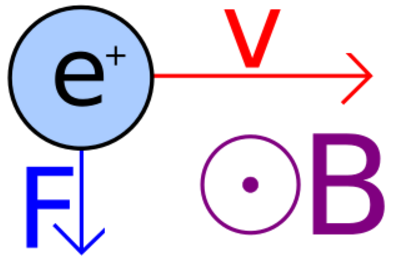
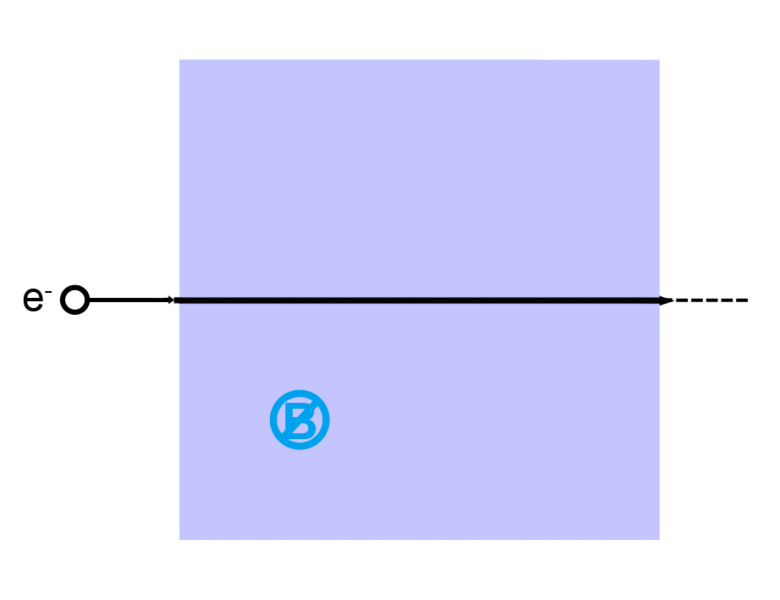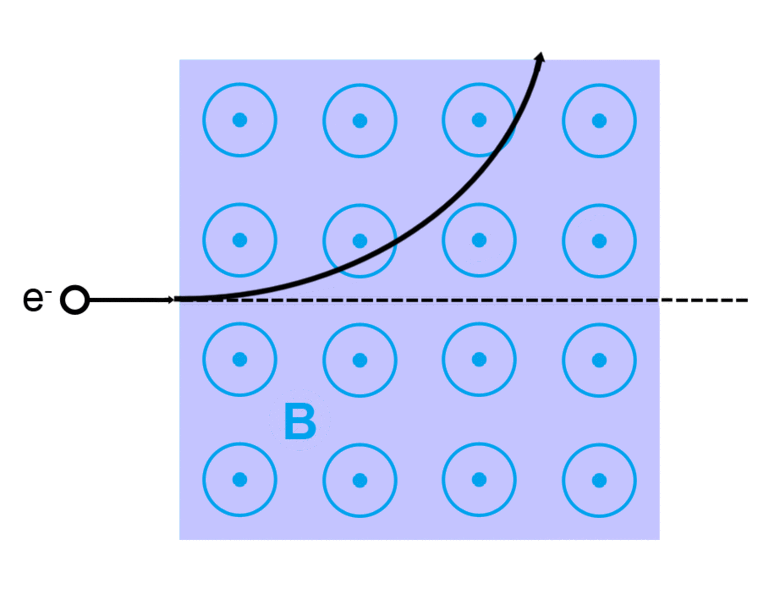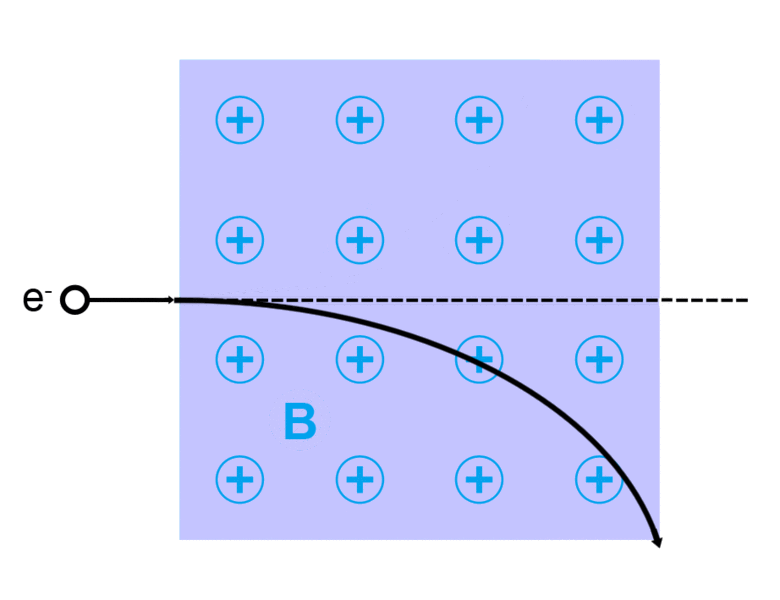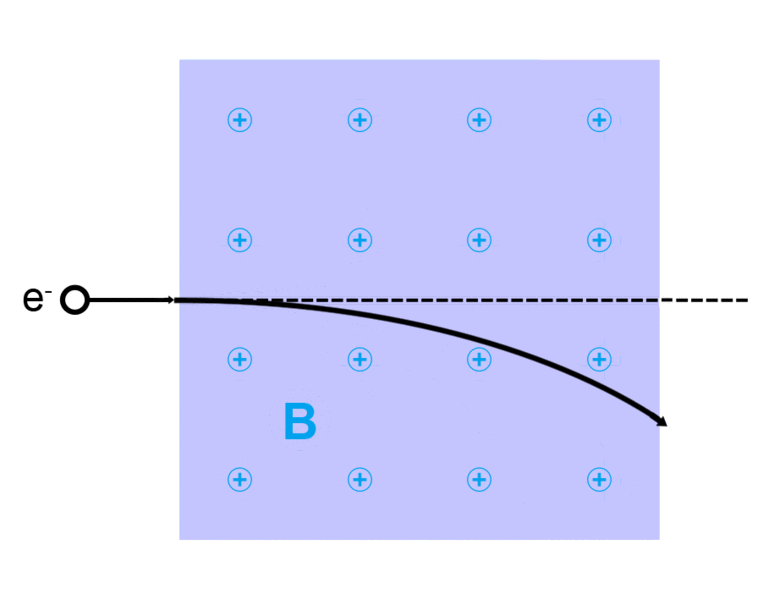
The direction of the magnetic force on a positive charge is given by the right hand rule. The force on a negative charge is in the opposite direction (the left hand rule).
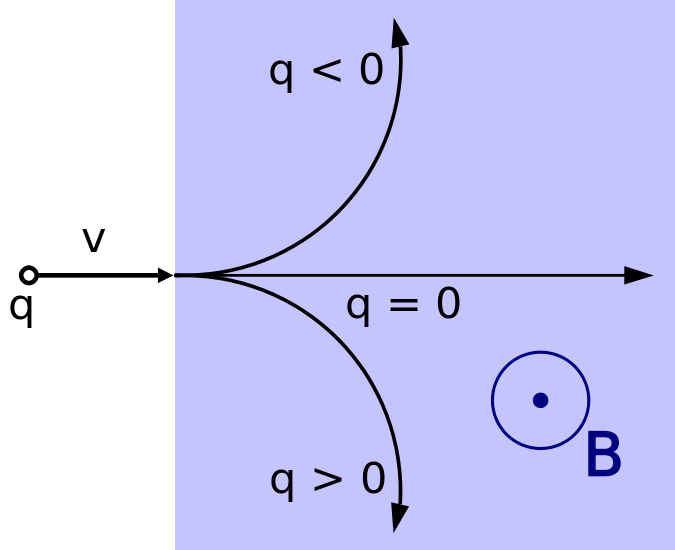 |
 |
 |
 |
|---|---|---|---|
 |
|---|
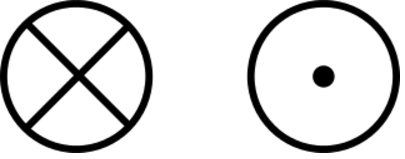
We use the above symbols to depict vectors in the Z direction. The vector on the left points into the plane and the vector on the right points out of the plane.
 |
 |
 |
|---|---|---|
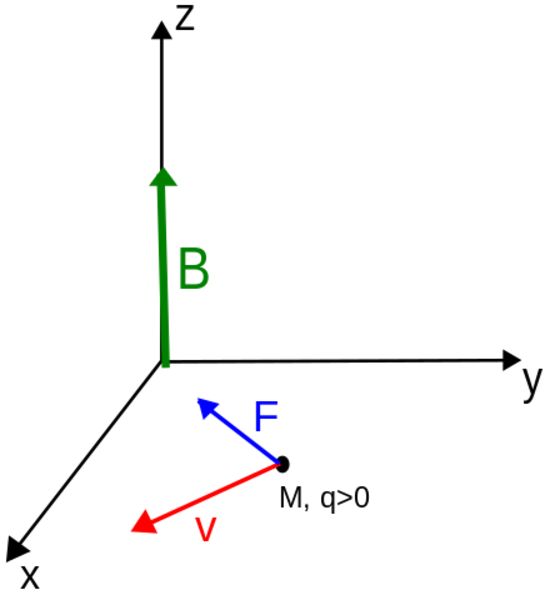
The direction of the force is the cross product "×" of V and B. The direction is given by the "right hand rule".
Magnetic field = B Magnetic force on a charge = F = Q V × B Magnetic force on a current = F = 2e-7 I × B
 |
|---|
Voltage = V Volts Capacitance = C Farads Total energy = E = ½ C V2 Joules Effective = Ee = ¼ C V2 JoulesNot all of the energy in a capacitor is harnessable because the voltage diminishes as the charge diminishes, hence the effective energy is less than the total energy.
 |
|---|
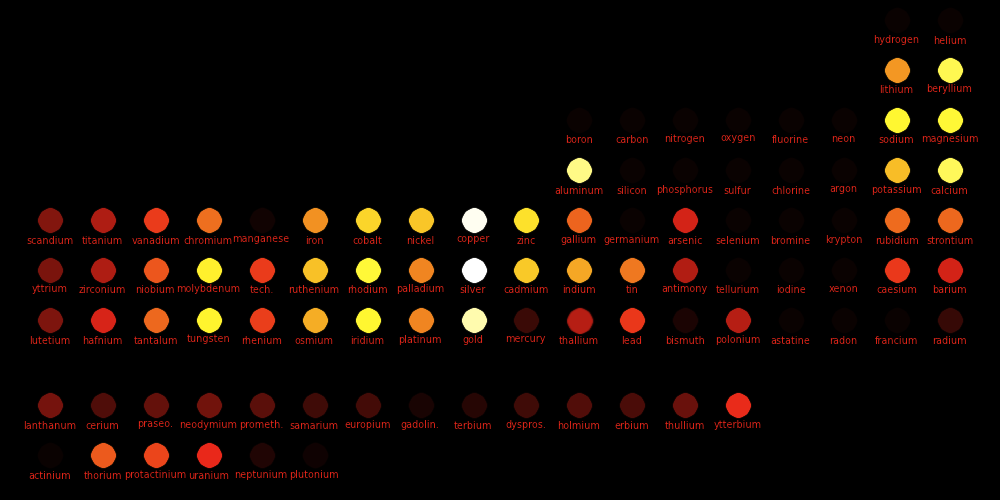
White: High conductivity Red: Low conductivity
Electric Thermal Density Electric C/Ct Heat Heat Melt $/kg Young Tensile Poisson Brinell
conduct conduct conduct/ cap cap number hardness
(e7 A/V/m) (W/K/m) (g/cm^3) Density (AK/VW) (J/g/K) (J/cm^3K) (K) (GPa) (GPa) (GPa)
Silver 6.30 429 10.49 .60 147 .235 2.47 1235 590 83 .17 .37 .024
Copper 5.96 401 8.96 .67 147 .385 3.21 1358 6 130 .21 .34 .87
Gold 4.52 318 19.30 .234 142 .129 2.49 1337 24000 78 .124 .44 .24
Aluminum 3.50 237 2.70 1.30 148 .897 2.42 933 2 70 .05 .35 .245
Beryllium 2.5 200 1.85 1.35 125 1.825 3.38 1560 850 287 .448 .032 .6
Magnesium 2.3 156 1.74 1.32 147 1.023 1.78 923 3 45 .22 .29 .26
Iridium 2.12 147 22.56 .094 144 .131 2.96 2917 13000 528 1.32 .26 1.67
Rhodium 2.0 150 12.41 .161 133 .243 3.02 2237 13000 275 .95 .26 1.1
Tungsten 1.89 173 19.25 .098 137 .132 2.54 3695 50 441 1.51 .28 2.57
Molybdenum 1.87 138 10.28 .182 136 .251 2896 24 330 .55 .31 1.5
Cobalt 1.7 100 8.90 .170 .421 1768 30 209 .76 .31 .7
Zinc 1.69 116 7.14 .388 693 2 108 .2 .25 .41
Nickel 1.4 90.9 8.91 .444 1728 15
Ruthenium 1.25 117 12.45 2607 5600
Cadmium 1.25 96.6 8.65 594 2 50 .078 .30 .20
Osmium 1.23 87.6 22.59 .130 3306 12000
Indium 1.19 81.8 7.31 430 750 11 .004 .45 .009
Iron 1.0 80.4 7.87 .449 1811 211 .35 .29 .49
Palladium .95 71.8 1828
Tin .83 66.8 505 22 47 .20 .36 .005
Chromium .79 93.9 .449 2180
Platinum .95 .133 2041
Tantalum .76 .140 3290
Gallium .74 303
Thorium .68
Niobium .55 53.7 2750
Rhenium .52 .137 3459
Vanadium .5 30.7 2183
Uranium .35
Titanium .25 21.9 .523 1941
Scandium .18 15.8 1814
Neodymium .156 1297
Mercury .10 8.30 .140 234
Manganese .062 7.81 1519
Germanium .00019 1211
Diamondiso 10 3320
Diamond e-16 2200 .509
Nanotube 10 3500 Carbon nanotube. Electric conductivity = e-16 laterally
Tube bulk 200 Carbon nanotubes in bulk
Graphene 10 5000
Graphite 2 400 .709 Natural graphite
Al Nitride e-11 180
Brass 1.5 120
Steel 45 Carbon steel
Bronze .65 40
Steel Cr .15 20 Stainless steel (usually 10% chromium)
Quartz (C) 12 Crystalline quartz. Thermal conductivity is anisotropic
Quartz (F) e-16 2 Fused quartz
Granite 2.5
Marble 2.2
Ice 2
Concrete 1.5
Limestone 1.3
Soil 1
Glass e-12 .85
Water e-4 .6
Seawater 1 .6
Brick .5
Plastic .5
Wood .2
Wood (dry) .1
Plexiglass e-14 .18
Rubber e-13 .16
Snow .15
Paper .05
Plastic foam .03
Air 5e-15 .025
Nitrogen .025 1.04
Oxygen .025 .92
Silica aerogel .01
Siemens: Amperes^2 Seconds^3 / kg / meters^2 = 1 Ohm^-1
For most metals,
Electric conductivity / Thermal conductivity ~ 140 J/g/K
Teslas
Field generated by brain 10-12
Wire carrying 1 Amp .00002 1 cm from the wire
Earth magnetic field .0000305 at the equator
Neodymium magnet 1.4
Magnetic resonance imaging machine 8
Large Hadron Collider magnets 8.3
Field for frog levitation 16
Strongest electromagnet 32.2 without using superconductors
Strongest electromagnet 45 using superconductors
Neutron star 1010
Magnetar neutron star 1014
The critical electric field for electric breakdown for the following materials is:
MVolt/meter
Air 3
Glass 12
Polystyrene 20
Rubber 20
Distilled water 68
Vacuum 30 Depends on electrode shape
Diamond 2000
Relative permittivity is the factor by which the electric field between charges is decreased relative to vacuum. Relative permittivity is dimensionless. Large permittivity is desirable for capacitors.
Relative permittivity
Vacuum 1 (Exact)
Air 1.00059
Polyethylene 2.5
Sapphire 10
Concrete 4.5
Glass ~ 6
Rubber 7
Diamond ~ 8
Graphite ~12
Silicon 11.7
Water (0 C) 88
Water (20 C) 80
Water (100 C) 55
TiO2 ~ 150
SrTiO3 310
BaSrTiO3 500
Ba TiO3 ~ 5000
CaCuTiO3 250000
A ferromagnetic material amplifies a magnetic field by a factor called the "relative permeability".
Relative Magnetic Maximum Critical
permeability moment frequency temperature
(kHz) (K)
Metglas 2714A 1000000 100 Rapidly-cooled metal
Iron 200000 2.2 1043
Iron + nickel 100000 Mu-metal or permalloy
Cobalt + iron 18000
Nickel 600 .606 627
Cobalt 250 1.72 1388
Carbon steel 100
Neodymium magnet 1.05
Manganese 1.001
Air 1.000
Superconductor 0
Dysprosium 10.2 88
Gadolinium 7.63 292
EuO 6.8 69
Y3Fe5O12 5.0 560
MnBi 3.52 630
MnAs 3.4 318
NiO + Fe 2.4 858
CrO2 2.03 386
Resistivity in 10^-9 Ohm Meters
293 K 300 K 500 K
Beryllium 35.6 37.6 99
Magnesium 43.9 45.1 78.6
Aluminum 26.5 27.33 49.9
Copper 16.78 17.25 30.9
Silver 15.87 16.29 28.7
Viscosity is analogous to electrical conductivity and thermal conductivity.
Suppose you measure the power exerted in climbing a set of stairs.
*) Description of the variable
Most units-style calculations can be done with this recipe.
If a smartphone is being used to play League of Legends, typical values for the
lithium battery are
It takes .7 kg of rice to feed one human for one day.
The price of electricity is 5 cents per kiloWatt hour.
The kinetic energy of an object in orbit is 32 MJoules/kg, and it costs
.44 dollars for this much energy in electricity. The real launch cost is
2000 dollars/kilogram.
A typical bottle of beer has a volume of 12 ounces, is 5% alcohol, and contains
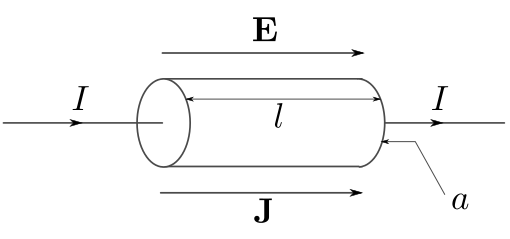
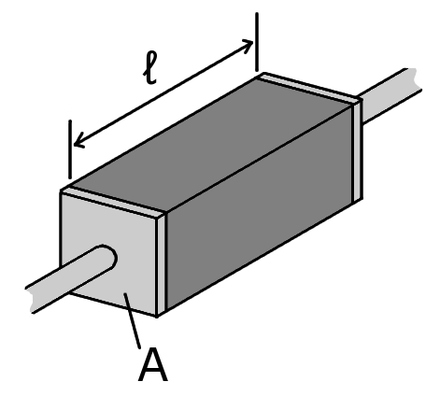
Electric quantities | Thermal quantities
|
Q = Charge Coulomb | Etherm= Thermal energy Joule
I = Current Amperes | Itherm= Thermal current Watts
E = Electric field Volts/meter | Etherm= Thermal field Kelvins/meter
C = Electric conductivity Amperes/Volt/meter | Ctherm= Thermal conductivity Watts/meter/Kelvin
A = Area meter^2 | A = Area meter^2
Z = Distance meter | Z = Distance meter^2
J = Current flux Amperes/meter^2 | Jtherm= Thermal flux Watts/meter^2
= I / A | = Ittherm / A
= C * E | = Ctherm * Etherm
V = Voltage Volts | Temp = Temperature difference Kelvin
= E Z | = Etherm Z
= I R | = Itherm Rtherm
R = Resistance Volts/Ampere = Ohms | Rtherm= Thermal resistance Kelvins/Watt
= Z / (A C) | = Z / (A Ct)
H = Current heating Watts/meter^3 |
= E J |
P = Current heating power Watts |
= E J Z A |
= V I |
Continuum quantity Macroscopic quantity
E <-> V
C <-> R = L / (A C)
J = C E <-> I = V / R
H = E J <-> P = V I
Quantity Electricity Thermal Viscosity
Stuff Coulomb Joule Momentum
Stuff/volume Coulomb/m^3 Joule/m^3 Momentum/m^3
Flow = Stuff/time Coulomb/second Joule/s Momentum/s
Potential Volts Kelvin Momentum/m^3
Field Volts/meter Kelvins/meter Momentum/m^3/m
Flow density = Flow/m^2 Amperes/meter^2 Watts/meter^2 Momentum/s/m^2
Conductivity Amperes/Volt/meter Watts/meter/Kelvin m^2/s
Resistance Volts/Ampere Kelvins/Watt s/m^3
Flow density = Conductivity * Field
Flow = Potential / Resistance
Field = -Gradient(Potential)
Fluid density = ρ (kg/meter3)
Fluid velocity = V
Fluid momentum density = U = D V
Kinematic viscosity = νk (meters2 / second)
Dynamic viscosity = νd = ρ νk (Pascal seconds)
Lagrangian time deriv. = Dt
Dt U = ∇⋅(νd∇U)
Dt V = ∇⋅(νk∇V)
Dynamic Kinematic Density
viscosity viscosity (kg/m3)
(Pa s) (m2/s)
Hydrogen .00000876
Nitrogen .0000178
Air .0000183 .0000150 1.22
Helium .000019
Oxygen .0000202
Xenon .0000212
Acetone .00031
Benzine .00061
Water at 2 C .00167
Water at 10 C .00131 .0000010 1000
Water at 20 C .00100 1000
Water at 30 C .000798 1000
Water at 100 C .000282 1000
Mercury .00153 .00000012
Blood .0035
Motor oil .065
Olive oil .081
Honey 6
Peanut butter 250
Asthenosphere 7e19 Weak layer between the curst and mantle
Upper mantle .8e21
Lower mantle 1.5e21
1 Stokes = 1 cm2/s = 10-4 m2/s
Schmidt number = Momentum diffusivity / Mass diffusivity
Prandtl number = Momentum diffusivity / Thermal diffusivity
Magnetic Prandtl number = Momentum diffusivity / Magnetic diffusivity
Prandtl Schmidt
Air .7 .7
Water 7
Liquid metals << 1
Oils >> 1
Height of stairs = H = 20 meters
Time to climb stairs = T = 10 seconds
Vertical speed = V = H/T = 2 meters/second
Mass of climber = M = 100 kg
Gravity constant = g = 10 meters/second2
Gravity energy = E = MgH = 20000 Joules
Power = P = E/T = 2000 Watts
There is a row for each variable and there are 5 columns showing the properties
of each variable. The columns are:
*) Symbol for the variable
*) Units-style equation
*) Numerical example
*) Units
Energy = E = = 20000 Joules (Typical smartphone battery energy)
Lifetime = T = = 14400 seconds (While playing League of Legends)
Power = P = E/T = 1.39 Watts
Mass = M = .027 kg
Energy/Mass = e = E/M = .75 MJoules/kg (Typical value for lithium batteries)
Power/Mass = p = P/M = 52 Watts/kg
The maximum power/mass that a lithium battery is capable of producing is
750 Watts/kg.
Energy in 1 Calorie = 4200 Joules
Time = T =86400 seconds = 1 day
Food energy in one day = E = 10.5 MJoules = 2500 Calories
Power = P = E/T = 121.5 Watts
Rice mass = M = .7 kg
Rice energy/mass = e = E/M = 15 MJoules/kg
In this example we added a column for non-SI units (days and Calories), which
have to be converted to SI units.
Power = P = 1000 Watts = 1 kiloWatt
Time = T = 3600 seconds = 1 hour
Energy = E = PT = 3.6 MJoules
Price = C = E/c = .05 $
Energy/$ = c = 72 MJoules/$
Orbital velocity = V =8000 meters/second
Mass = M = 1 kg
Kinetic energy = E = ½MV2 = 32 MJoules
Electricity energy/$ = c = 72 MJoules/$
Electricity cost = C = E/c = .44 $




.6 ounces of alcohol. We use this amount as a reference unit and define
.6 ounces of alcohol to be one "Bond".
Volume of the drink = V
Fraction of alcohol = F
Volume of alcohol = Valc = F V
Volume of one beer = Vbeer = 12 ounces
Alcohol fraction of beer = Fbeer = .05
Alcohol volume in one beer = VBond = .6 ounces
One "Bond" of alcohol = .6 ounces
One wine or Scotch bottle = 25.4 ounces = 750 ml
One ounce = 29.6 mL
Alcohol Volume Alcohol Alcohol $ $/Bond
fraction (oz) (oz) (Bonds)
Beer (12 oz) .05 12 .6 1 .67 .67 Budweiser
Wine glass .13 4.6 .6 1 8 8.0 Napa Valley
Scotch shot .40 1.5 .6 1 8 8.0 Laphroaig
Beer pitcher .05 64 3.2 5.3 16 3.0 Budweiser
Beer keg .05 1984 99.2 165.3 100 .60 Budweiser
Wine bottle .13 25.4 3.3 5.5 3 .55 Charles Shaw
Vodka bottle .40 25.4 10.1 16.9 15 .89 Smirnoff
Scotch bottle .40 25.4 10.1 16.9 50 3.0 Laphroaig
Distilled ethanol .95 25.4 24.1 40.2 15 .37 Everclear