MJoule/kg Calories/gram
Sugar 17 5
Protein 17 5
Alcohol 25 7
Fat 38 9
ATP .057
Phosphocreatine .137
Hydrogen 143
Natural gas 53.6
Gasoline 47
Coal 24
Wood 16
Li-ion battery .6
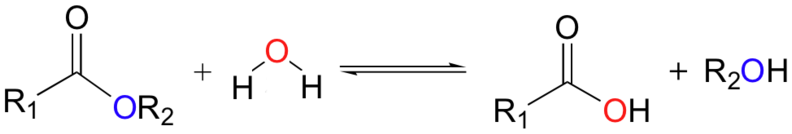
Hydrocarbons have good energy/mass and are good for energy storage. Sugars and fats are convenient hydrocarbons to metabolize, and humans can metabolize most of them.
 |
 |
 |
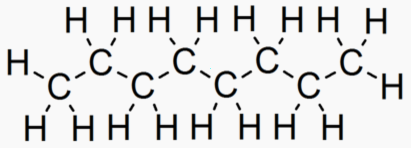 |
|---|---|---|---|
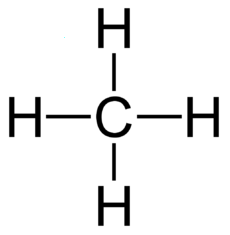
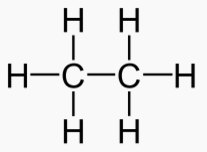
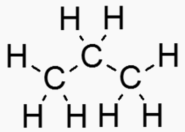
An "Alkane" is a carbon chain with hydrocarbons attached. At standard temperature (300 K), alkanes are solid if they have more than 20 carbons. This is why lipids (long alkanes) are the optimal form of energy storage. Short alkanes are liquids or gases at STP and are hard to store.
In the following table, the first section shows properties of alkanes and the second section shows properties of other energy sources.
Alkane Carbons Energy of Melt Boil Solid Liquid Gas Phase at
type combustion (K) (K) density density density 300 K
(MJ/kg) (g/cm^3) (g/cm^3) (g/cm^3)
Hydrogen 0 141.8 14.0 20.3 .07 .000090 Gas
Methane 1 55.5 90.7 111.7 .423 .00070 Gas
Ethane 2 51.9 90.4 184.6 .545 .0013 Gas
Propane 3 50.4 85.5 231.1 .60 .0020 Gas
Butane 4 49.5 136 274 .60 .0025 Gas
Pentane 5 48.6 143.5 309 .63 Liquid
Hexane 6 48.2 178 342 .65 Liquid
Heptane 7 48.0 182.6 371.5 .68 Liquid
Octane 8 47.8 216.3 398.7 .70 Liquid
Dodecain 12 46 263.5 489 .75 Liquid
Hexadecane 16 46 291 560 .77 Liquid
Icosane 20 46 310 616 .79 Solid
Alkane-30 30 46 339 723 .81 Solid
Alkane-40 40 46 355 798 .82 Solid
Alkane-50 50 46 364 848 .82 Solid
Alkane-60 60 46 373 898 .83 Solid
Gasoline ~ 8 47 .76 Liquid Mostly alkanes with ~ 8 carbons
Natural gas 54 91 112 Gas Mostly methane
Coal 32 - - Solid Mostly carbon
Wood 22 - - Solid Carbon, oxygen, hydrogen
Pure carbon 1 32.8 - - Solid Pure carbon, similar to coal
Methanol 1 175.6 337.8 .79 Liquid
Ethanol 2 159 351.5 .79 Liquid
Propanol 3 147 370 Liquid
An alkane with 7 or more carbons has a heat of combustion of 46 MJoules/kg.
A nitrogen molecule is more tightly bound than an oxygen molecule, making it impossible to extract energy from hydrocarbons with nitrogen. Few things burn in a nitrogen atmosphere, lithium and magnesium being examples.
 |
|---|
A sugar generally has the formula CN H2N ON, where N = 2, 3, etc. The common sugars are hexoses with N=6.
Number of Number of
carbons sugars
Diose 2 1
Triose 3 2
Tetrose 4 3
Pentose 5 4
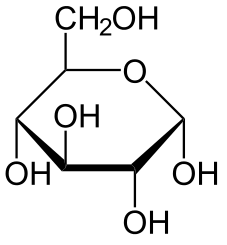
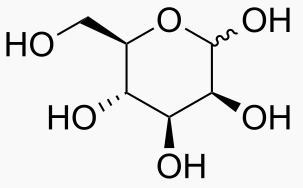
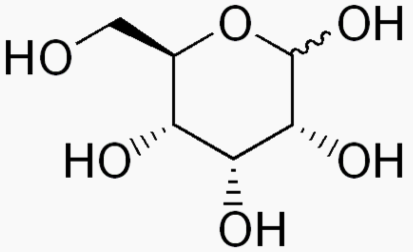
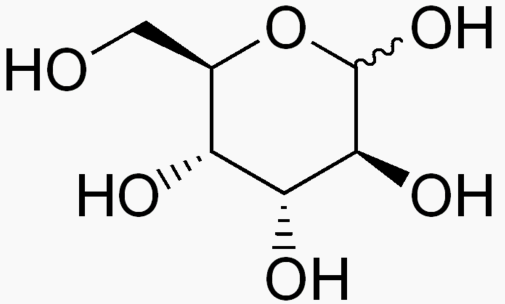
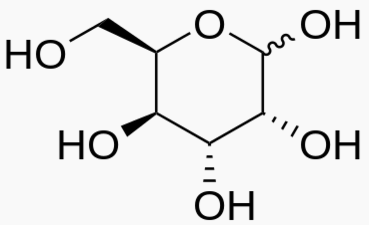
Hexose 6 12 At least 6 carbons are required to form a ring
Heptose 7 many Rarely observed in nature
Octose 8 many Unstable. Not observered in nature.
"Number of sugars" refers to the number of different types of sugar molecules for each
carbon number.
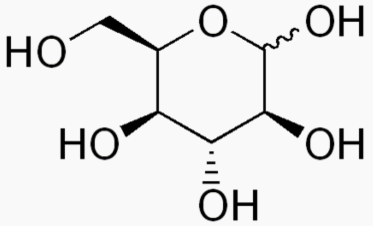
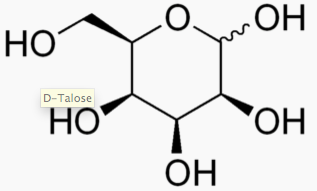
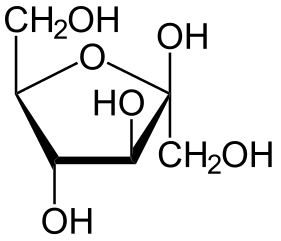
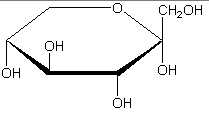
Each sugar molecule has two mirror-symmetric forms, the "D" and "L" form. Only the D forms are found in nature.
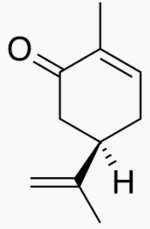
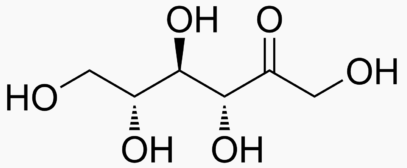
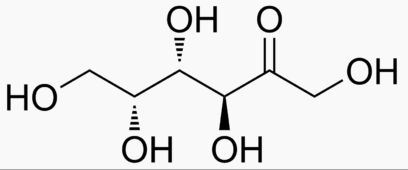
The following figures show all sugars up to 6 carbons. All can be metabolized by humans.
2 carbons:
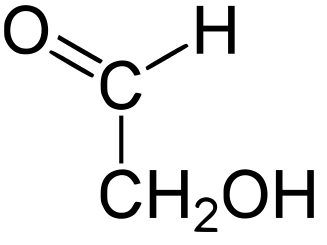 |
|---|
3 carbons:
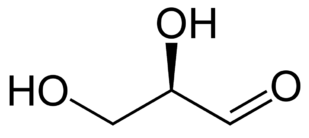 |
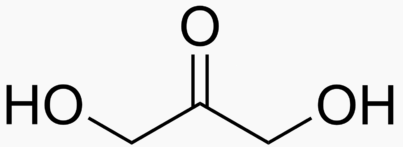 |
|---|---|
4 carbons:
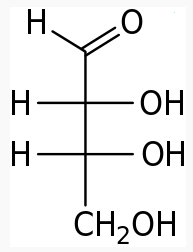 |
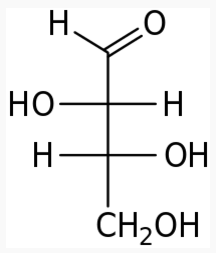 |
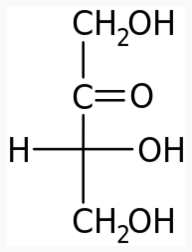 |
|---|---|---|
5 carbons:
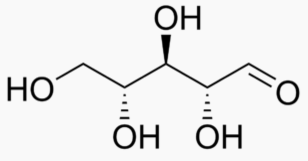 |
 |
 |
 |
|---|---|---|---|
6 carbons:
 |
 |
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|---|---|
 |
 |
 |
 |
|---|---|---|---|
Energy Sweetness
Succrose 1.00 1.00 Benchmark
Glucose .74
Maltose .32
Galactose .32
Lactose .16
Allose
Altrose
Mannose
Fructose 1.73
Psichose .70
Tagatose .38 .92
Sorbose 1.0
Honey .97
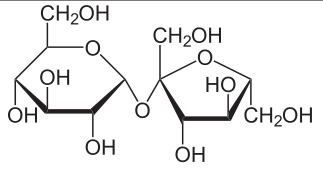
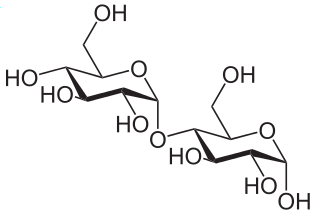
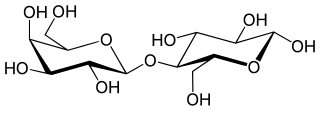
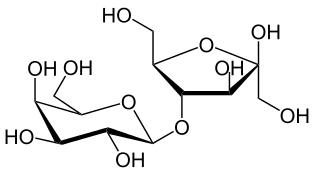
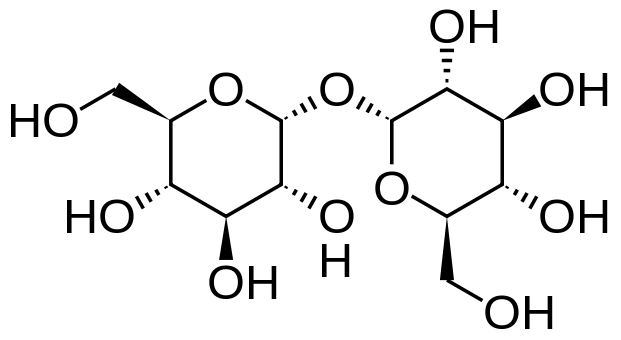
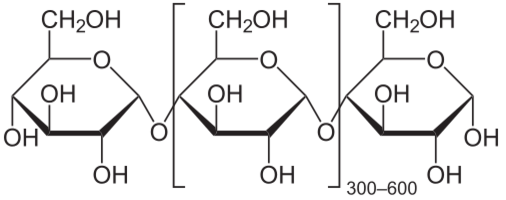
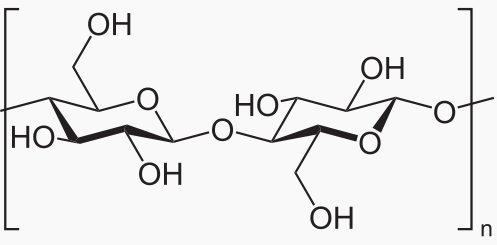
Monosaccharde: 1 sugar molecule Disaccharide: 2 monosaccharides Polysaccharide: More than 2 monosaccharides, such as starch and cellulose
 |
 |
 |
 |
 |
|---|---|---|---|---|
Sucrose = Glucose + Fructose Maltose = Glucose + Glucose Lactose = Galactose + Glucose Lactulose = Galactoce + Fructose Trehalose = Glucose + Glucose Cellobiose = Glucose + Glucose Chitobiose = Glucosamine + GlucosamineStarch and cellulose are long chains of glucose molecules.
 |
 |
|---|---|
 |
 |
|---|---|
 |
 |
 |
 |
 |
|
|---|---|---|---|---|---|
Fatty acids and sugars are metabolized in the following stages, with each stage yielding energy.
Fatty acid -> Acetyl -> CO2 and H2O Sugar -> Pyruvate -> CO2 and H2O
Blood delivers fatty acids to cells.
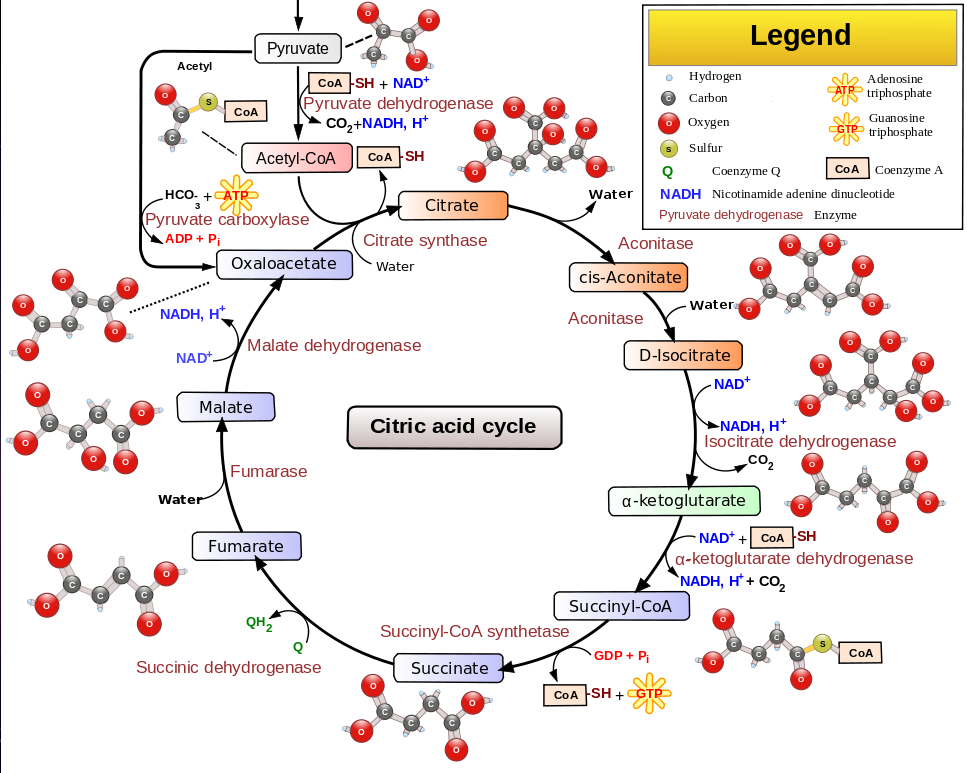
The citric acid cycle (Krebs cycle) converts acetyl or pyrovate into H2O and CO2. Coenzyme-A carries the acetyl around.
A fat molecule is converted into a fatty acid by lipolysis, and then the fatty acid is converted into acetyl by beta oxydation, and then the acetyl is converted into H2O and CO2 by the citric acid cycle.
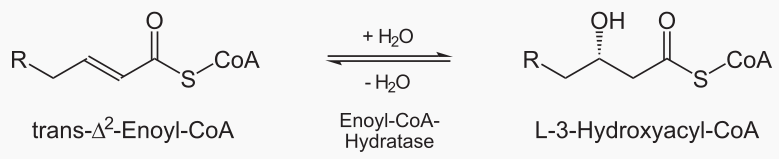
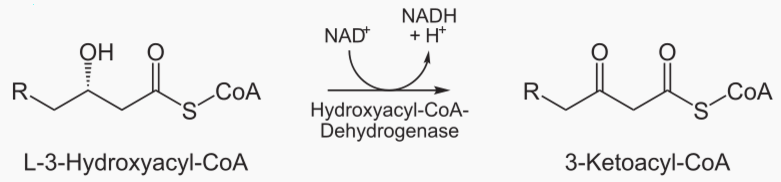
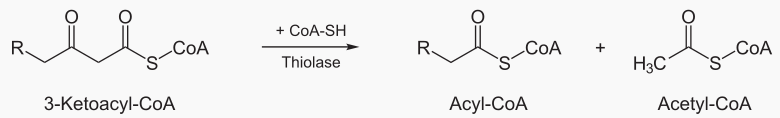
Beta oxidation cleaves 2 carbons from a fatty acid, which becomes acetyl. This process is repeated until te entire fatty acid has been converted into acetyls.
The steps of beta oxidation are:
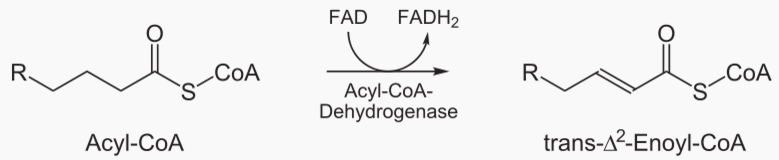 |
|---|
 |
|---|
 |
|---|
 |
|---|
Glycolysis converts a glucose molecule into 2 pyrovate molecules. A summary of the reaction showing only the starting and ending points is:
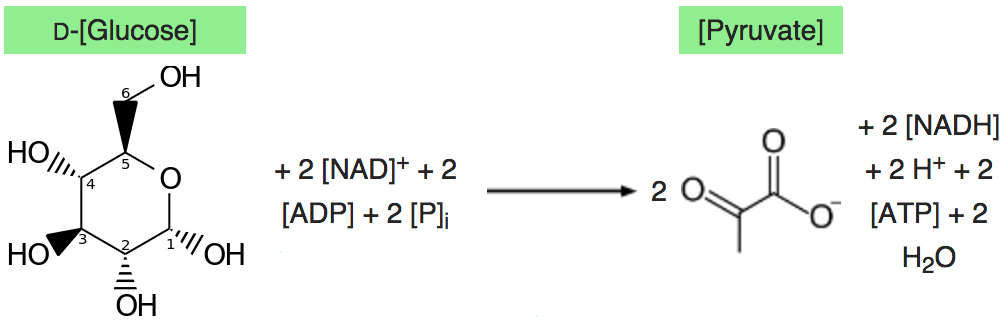 |
|---|
The full reaction is:
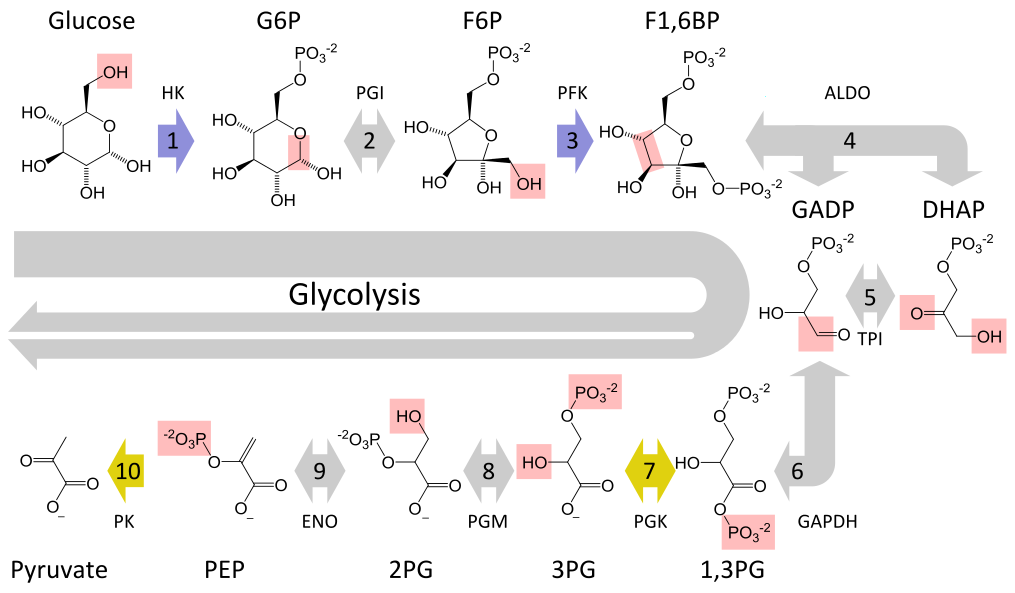 |
|---|
 |
|---|
 |
|---|
The citric acid cycle (Krebs cycle) converts acetyl or pyrovate into H2O and CO2.
Fat metabolism oxidizes a carbon chain so that the chain can be split into acetyl. The strategy of the citric acid cycle is to further oxidize the acetyl (now a part of citrate) so that the remaining carbon bonds in the acetyl can be broken.
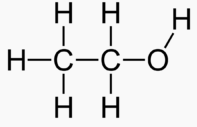
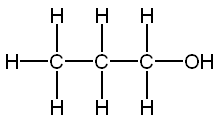
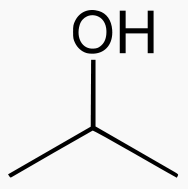
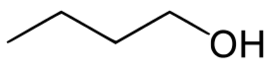
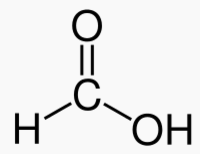
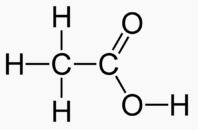
An alcohol is a carbon chain with one OH attached.
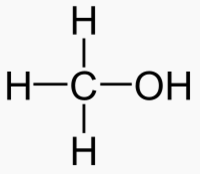 |
 |
 |
 |
 |
|---|---|---|---|---|
Carbons
Methanol 1 Toxic
Ethanol 2 Inebriating
Propanol 3 3 times more inebriating than ethanol
Isopropanol 3 Toxic
Butanol 4 6 times more inebriating than ethanol
 |
 |
 |
|---|---|---|
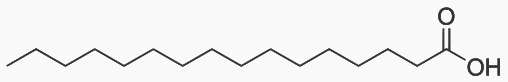
Palmitic acid has 16 carbons and is the most common fatty acid found in food.
Carbons 1 2 Vinegar 3 4 Found in butter 8 Found in coconuts 10 Found in coconuts 12 Found in coconuts 16 Most common fatty acid. Found in palm oil 18 Found in chocolate 20 Found in peanut oil
 |
 |
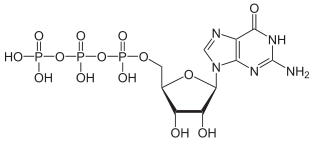 |
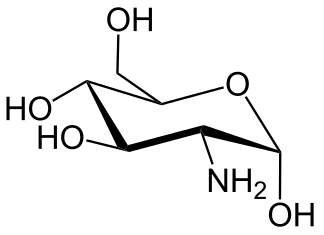 |
 |
 |
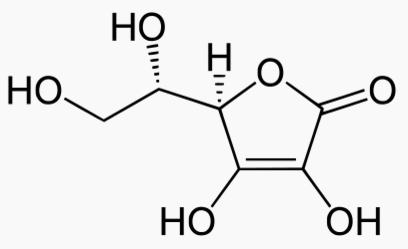 |
|---|---|---|---|---|---|---|
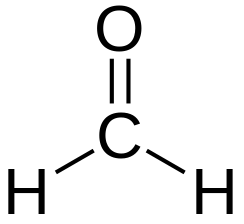 |
|---|
LD50
(mg/kg)
CO Carbon monoxide
HCN 6.4 Hydrogen cyanide
CH2O Methanol
CH2O Formaldehyde
H2S Hydrogen sulfide
NO2 Nitrite
Cl2 Chlorine
Fl2 Fluorine
Ethanol 7060
Salt 3000
Caffeine 192
Aspirin 200
NaNO2 180 Sodium nitrite
Cobalt 80
NaF 52
Capsaicin 47 Chili pepper
Mercury 41
Arsenic 13
Nicotine .8
Bromine
C2N2
PH3
SiCl4
Almost anything with fluorine or bromine is toxic.
Weakly toxic:
C2H2 Acetylene. Inebriating C3H6 Propene. Inebriating
Among the elements required for life, nitrogen is the scarcest.
The nitrogen in the first 250 km of the Earth's crust has the same mass as
the nitrogen in the atmosphere.
The elements that are abundant in the crust and never used by life are aluminum and titanium.
All elements necessary for life are abundant in either the crust, the ocean, or the atmosphere.
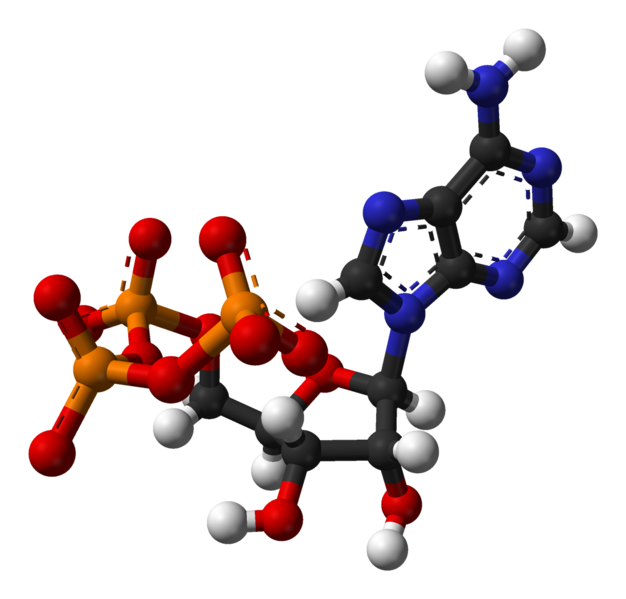
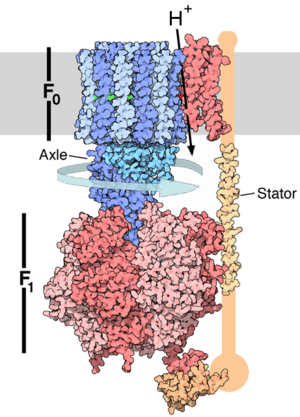
Enzymes use ATP as an energy source to power chemical reactions. ATP and ATP
synthase are common to all Earth life.
* Video of the ATP synthase enzyme in action
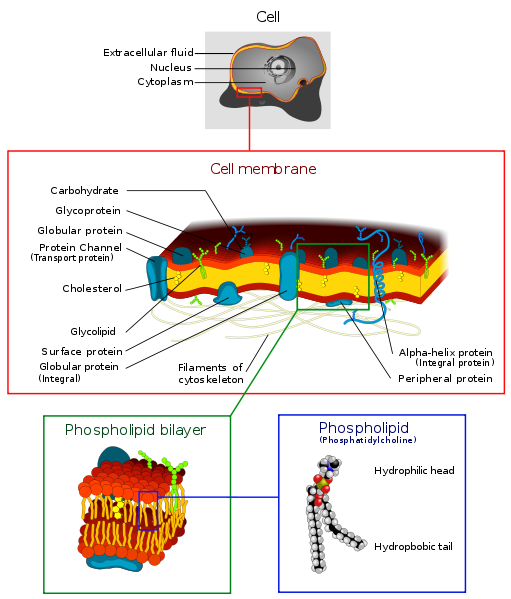
Cell walls are formed from a double layer of lipids. They are elastic and they
self-assemble.
Each lipid has a polar and a non-polar end. The polar end faces the water
and the non-polar end faces another lipid.
* Video of the self-assembly of a bilipid layer
If life were to exist in a non-polar solvent it would have to find another way
to make cell walls.
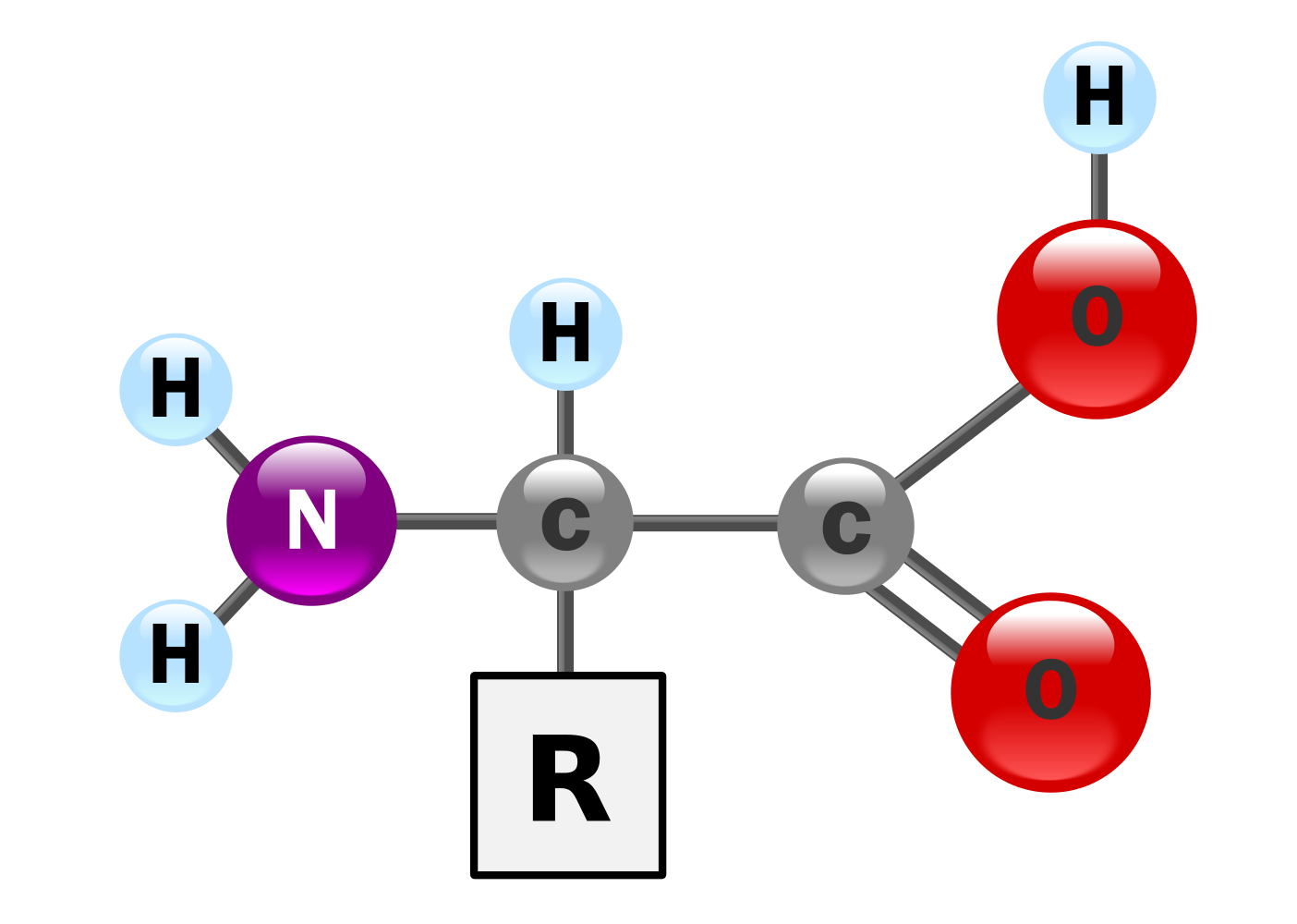
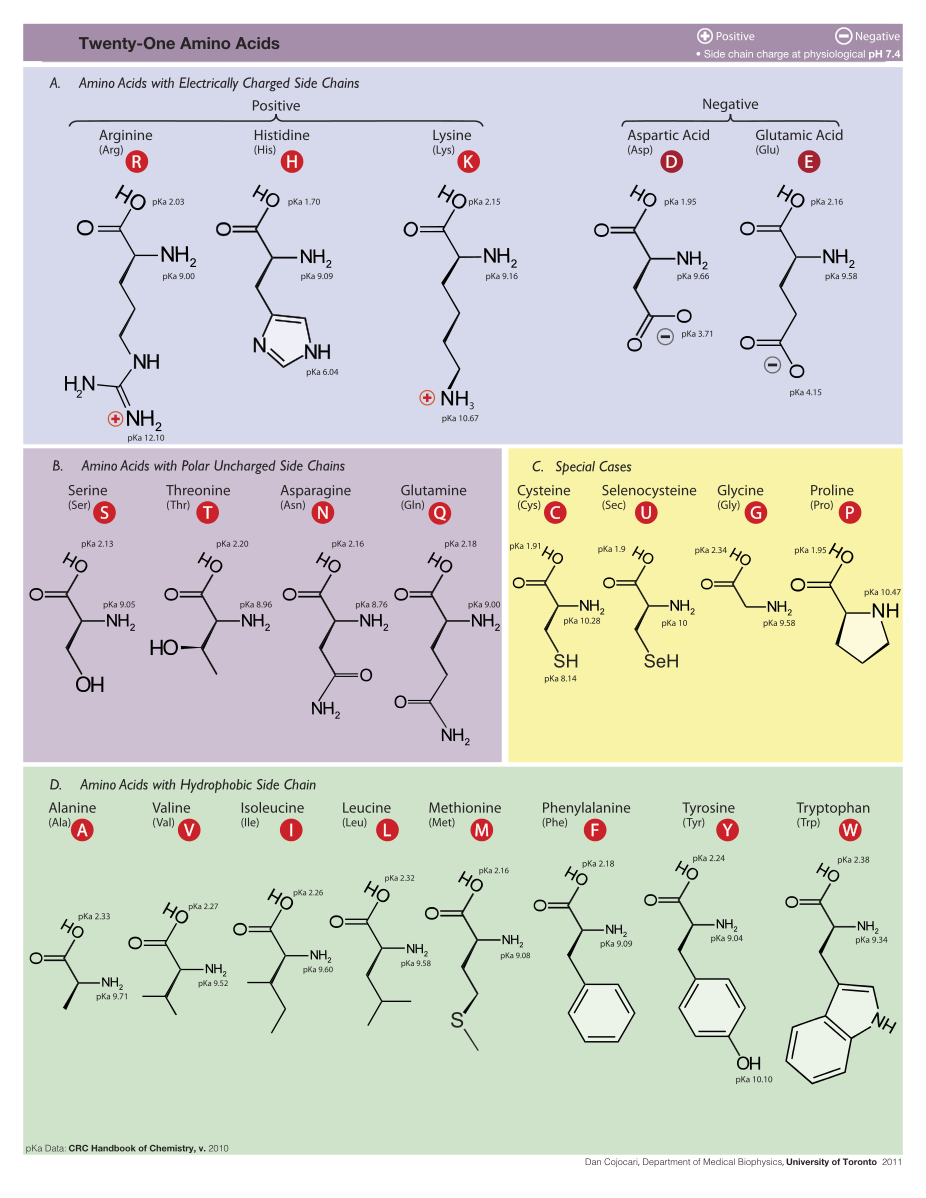
Amino acids have the above form, where R stands for an arbitrary molecule.
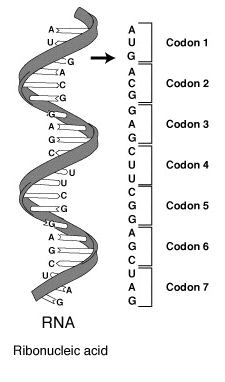
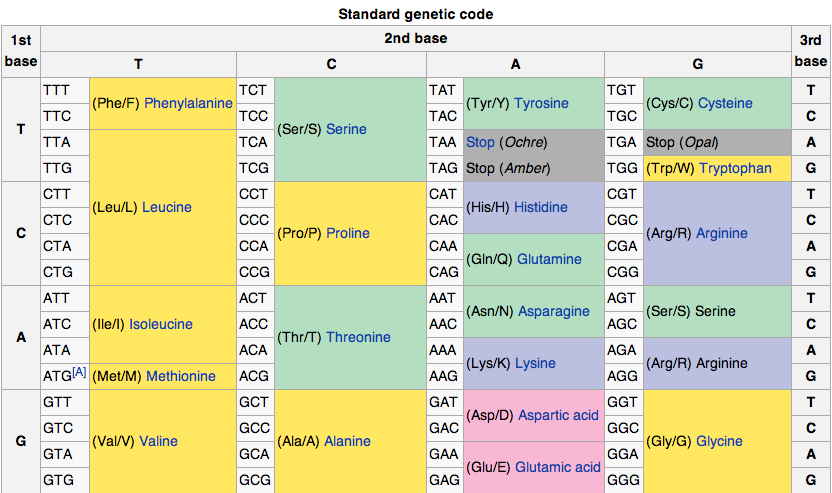
DNA codes a sequence of amino acids. The 64-element codon system is universal to
Earth life.
The codon ATG both codes for methionine and serves as an initiation site: the
first ATG in an mRNA's coding region is where translation into protein begins.
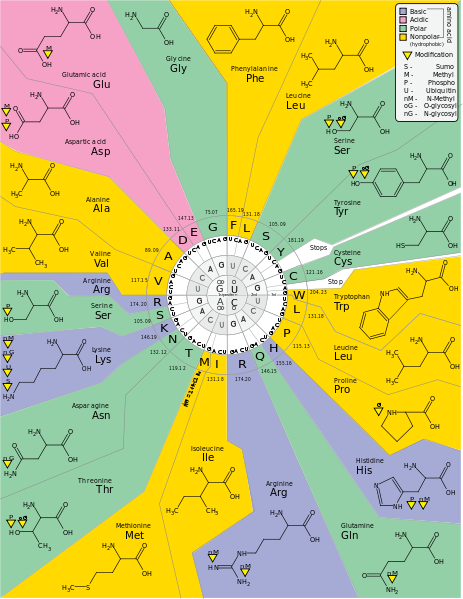
21 amino acids are used by eucaryote. More than 500 amino acids are known.
Minimum Used by Used Human Crust Ocean Atmosphere
for life humans by life ppt ppt ppt ppt
Hydrogen * * * .10 1.5 108 .00055
Helium .000008 .0052
Lithium .02
Beryllium .0028
Boron * * .0000007 .01
Carbon * * * .18 1.0 .028 .407
Nitrogen * * * .03 .02 780
Oxygen * * * .65 460 858 210
Fluorine * .5
Neon .0000051 .018
Sodium * * * .0015 25 10.8
Magnesium * * * .0005 25 1.3
Aluminum 82
Silicon * 275
Phosphorus * * * .011 1.1
Sulfur * * * .0025 .4 .91
Chlorine * * * .0015 .2 19
Argon .0035 9.3
Potassium * * * .0025 20 .4
Calcium * * * .014 45 .4
Scandium .022
Titanium 5.6
Vanadium * .12
Chromium .10
Manganese * * .00000017 .95
Iron * * .00006 60
Cobalt * * .000000021 .025
Nickel * .084
Copper * * .000001 .06
Krypton .0011
Zinc * * .000032 .075
Gallium .019
Germanium .0015
Arsenic * .0018
Selenium * * .00000019 .00005
Bromine * * .0000029 .0024 .067
Molybdenum * .0012
Tellurium * .000001
Iodine * * .00000016 .00045
Tungsten * .0012
* Video of an amoeba
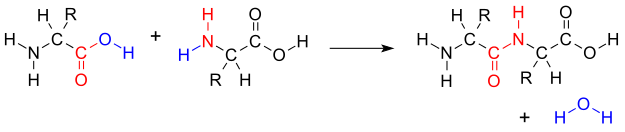
Synthesis of two amino acids. Proteins are chains of animo acids with a backbone of
the form:
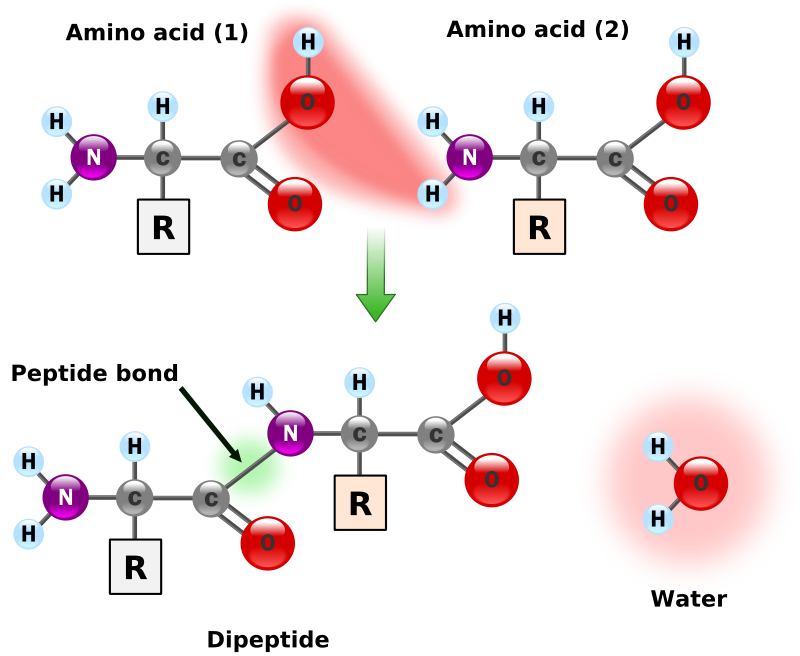
C-C-N-C-C-N-C-C-N-C-C-N-C-C-N



































































































Hydrogen White
Carbon Black
Nitrogen Blue
Oxygen Red
Sulfur Yellow
Scoville scale (relative capsaicin content)
Ghost pepper 1000000
Trinidad 1000000 Trinidad moruga scorpion
Naga Morich 1000000
Habanero 250000
Cayenne pepper 40000
Malagueta pepper 40000
Tabasco 40000
Jalapeno 5000
Guajillo pepper 5000
Cubanelle 500
Banana pepper 500
Bell pepper 50
Pimento 50
Molecule Relative hotness
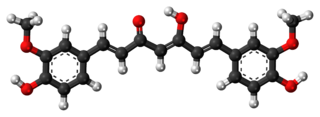
Rresiniferatoxin 16000
Tinyatoxin 5300
Capsaicin 16 Chili pepper
Nonivamide 9.2 Chili pepper
Shogaol .16 Ginger
Piperine .1 Black pepper
Gingerol .06 Ginger
Capsiate .016 Chili pepper