 |
|---|
For gases, the density at boiling point is used. Size data
 |
|---|
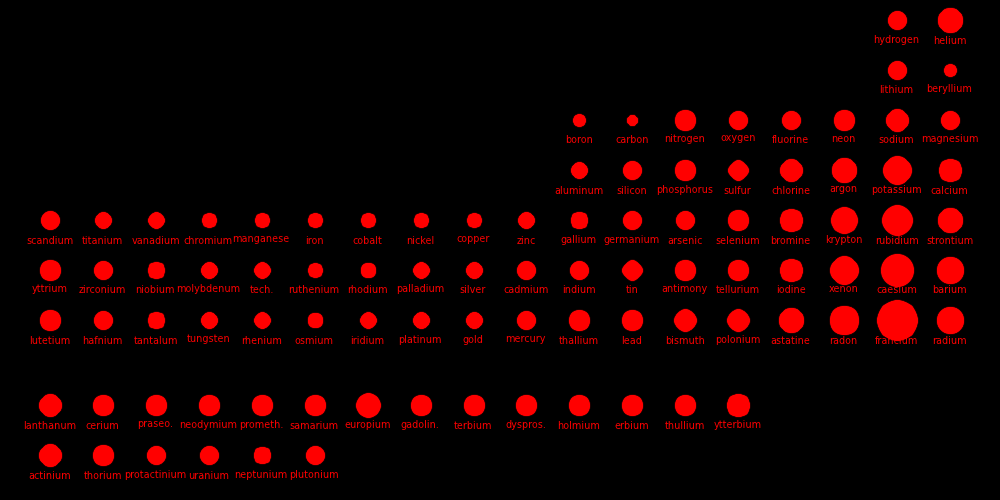
Copper atoms stack like cannonballs. We can calculate the atom size by assuming the atoms are shaped like either cubes or spheres. For copper atoms,
Density = D = 8900 kg/m3 Mass = M = 9.785e-26 kg Number density = N = D / M = 9.096e28 atoms/m3 Cube volume = Υcube= 1 / N = 1.099e-29 m3 Volume/atom if the atoms are cubes Cube length = L = Υ1/3cube = 2.22e-10 m Side length of the cube Sphere fraction = f = π/(3√2) = .7405 Fraction of volume occupied by spheres in a stack o spheres Sphere volume = Υsph = Υcube f = 8.138e-30 m3 = 4⁄3πR3 Volume/atom if the atoms are spheres Sphere radius = R = 1.25e-10 m
 |
|---|
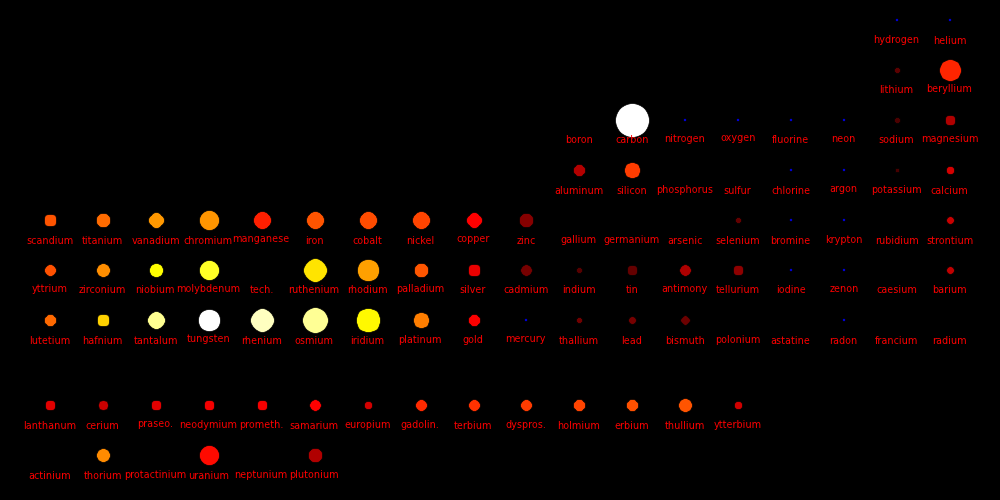
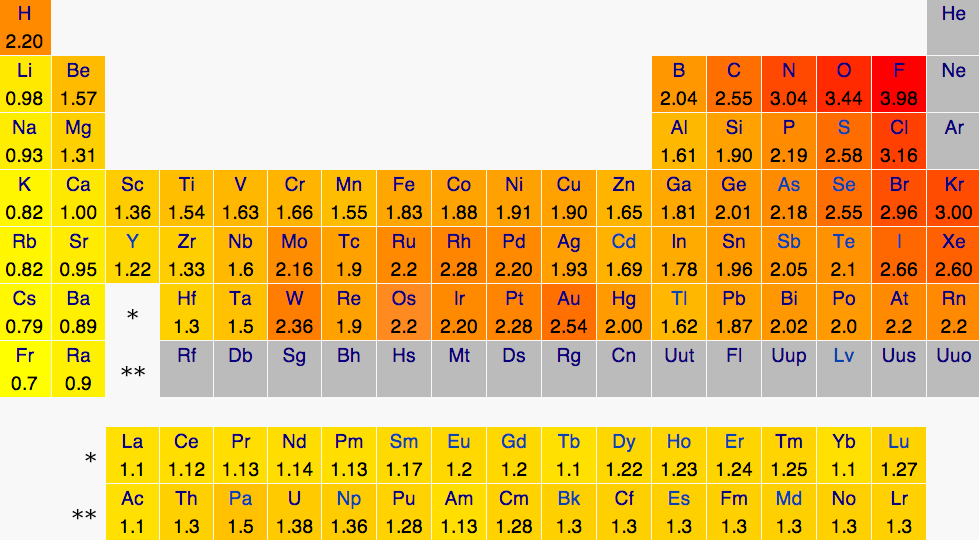
Dot size = (Shear Modulus)1/3 A measure of a material's strength
Dot color = Melting point
White = Highest melting points
Red = Lowest melting points
Blue = Elements that are a gas or a liquid at room temperature and pressure.
Liquids and gases have a shear modulus of 0.
Rocket nozzles are made from materials with a high melting point, a high shear
strength, and a high atomic mass. Tungsten is the element of choice,
especially because it's vastly cheaper than Rhenium, Osmium, and Iridium.
For carbon, the values are given for diamond form.
 |
|---|
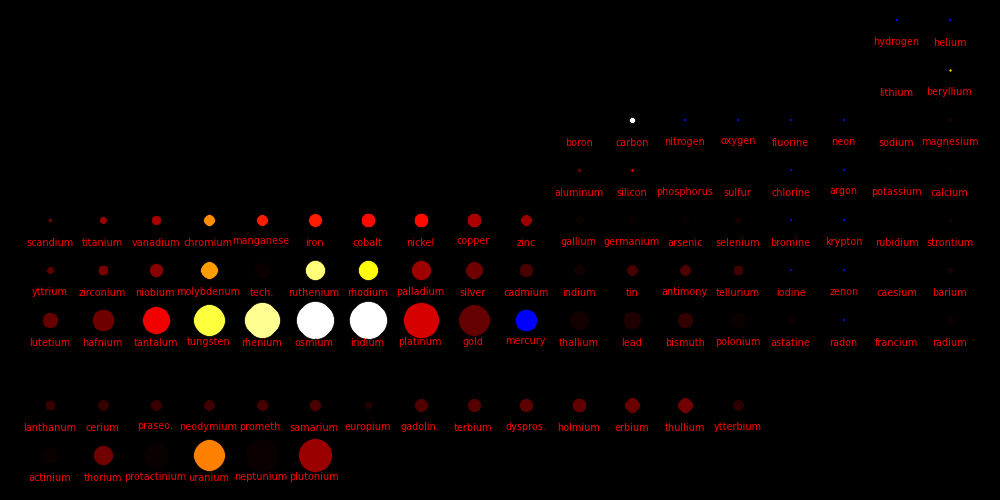
Color = Shear Modulus Dot size = DensityShear data Density data
 |
|---|
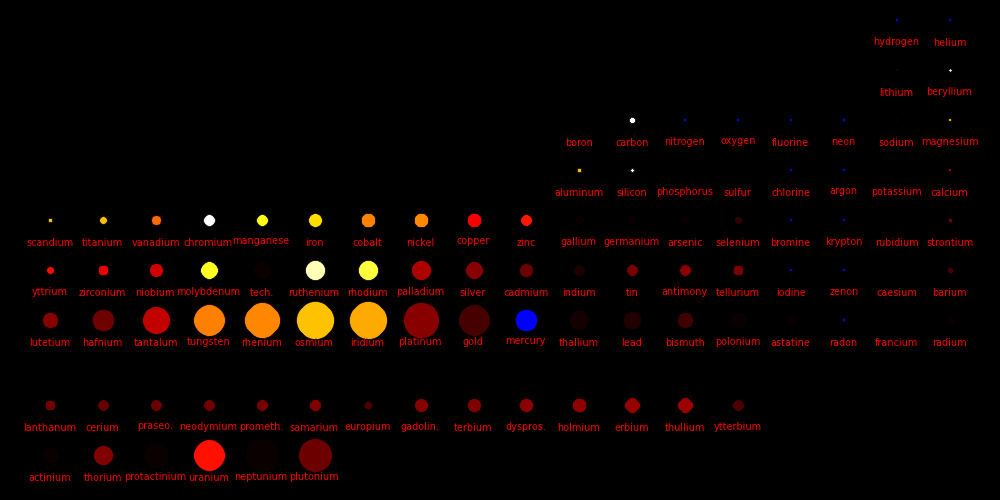
Color = Shear Modulus / Density A measure of a material's "strength to weight" ratio Dot size = DensityThe metals with the highest strength to weight ratio are Chromium, Ruthenium, and Beryllium.
Chromium is common in the Earth's crust and Ruthenium is rare.
Density Melt Boil Young Young $/kg ppm in metallic
g/cm^3 K K GPa /rho asteroid
Tungsten 19.25 3693 5828 411 21.4 50 ~ 1
Rhenium 21.0 3459 5869 463 22.0 4600 ~ 1
Osmium 22.59 3306 5285 550 24.3 12000 2
Tantalum 16.7 3290 5731 186 11.1 400 ~ .5
Molybdenum 10.28 2896 4912 329 31.0 21 ~10
Niobium 8.75 2750 5017 105 12.0 40 ~ 3
Iridium 22.4 2739 4701 528 23.6 14000 2
Ruthenium 12.45 2607 4423 447 35.9 5500 5
Hafnium 13.31 2506 4876 78 5.9 500 ~ 1
Rhodium 12.41 2237 3968 380 30.6 90000 2
Thorium 11.7 2115 5061 79 6.8 ~ .5
Platinum 21.45 2041 4098 168 7.8 55000 9
Vanadium 6.0 2183 3680 128 21.3 0
Chromium 7.19 2180 2944 279 38.8 0
Zirconium 6.52 2128 4682 88 14.5 0
Titanium 4.51 1941 3560 116 25.7 0
Palladium 12.02 1828 3236 121 10.1 ~ ?
Iron 7.87 1811 3134 211 26.8 100000
Nickel 8.91 1728 3186 200 22.4 10000
Cobalt 8.90 1768 3200 209 23.5 ~ 1000
Uranium 19.1 1405 4404 208 10.9 1
Beryllium 1.85 1560 2742 287 155.1 0
Manganese 7.21 1519 2334 198 27.5 0
Aluminum 2.70 933 2792 70 25.9 0
Magnesium 1.74 923 1363 45 25.9 0
 |
|---|
Red: Lowest conductivities White: Highest conductivitiesIf an element's conductivity is more than 2 orders of magnitude less than that of silver then it is left blank.
Earliest Shear Melt Density
known use Modulus (K) (g/cm^3)
(year) (GPa)
Wood < -10000 15 - .9
Rock < -10000
Carbon < -10000 -
Diamond < -10000 534 3800 3.5
Gold < -10000 27 1337 19.3
Silver < -10000 30 1235 10.5
Sulfur < -10000
Copper -9000 48 1358 9.0
Lead -6400 6 601 11.3
Brass -5000 ~40 Copper + Zinc
Bronze -3000 ~40 Copper + Tin
Tin -3000 18 505 7.3
Antimony -3000 20 904 6.7
Mercury -2000 0 234 13.5
Iron -1200 82 1811 7.9
Arsenic 1649 8 1090 5.7
Cobalt 1735 75 1768 8.9 First metal discovered since iron
Platinum 1735 61 2041 21.4
Zinc 1746 43 693 7.2
Tungsten 1783 161 3695 19.2
Chromium 1798 115 2180 7.2
Stone age antiquity
Copper age -9000
Bronze age -3500
Iron age -1200
Bronze holds an edge better than copper and it is more corrosion resistant.
Gold was the densest known element until the discovery of platinum in 1735. This made it impossible to counterfeit as a currency.
 |
|---|
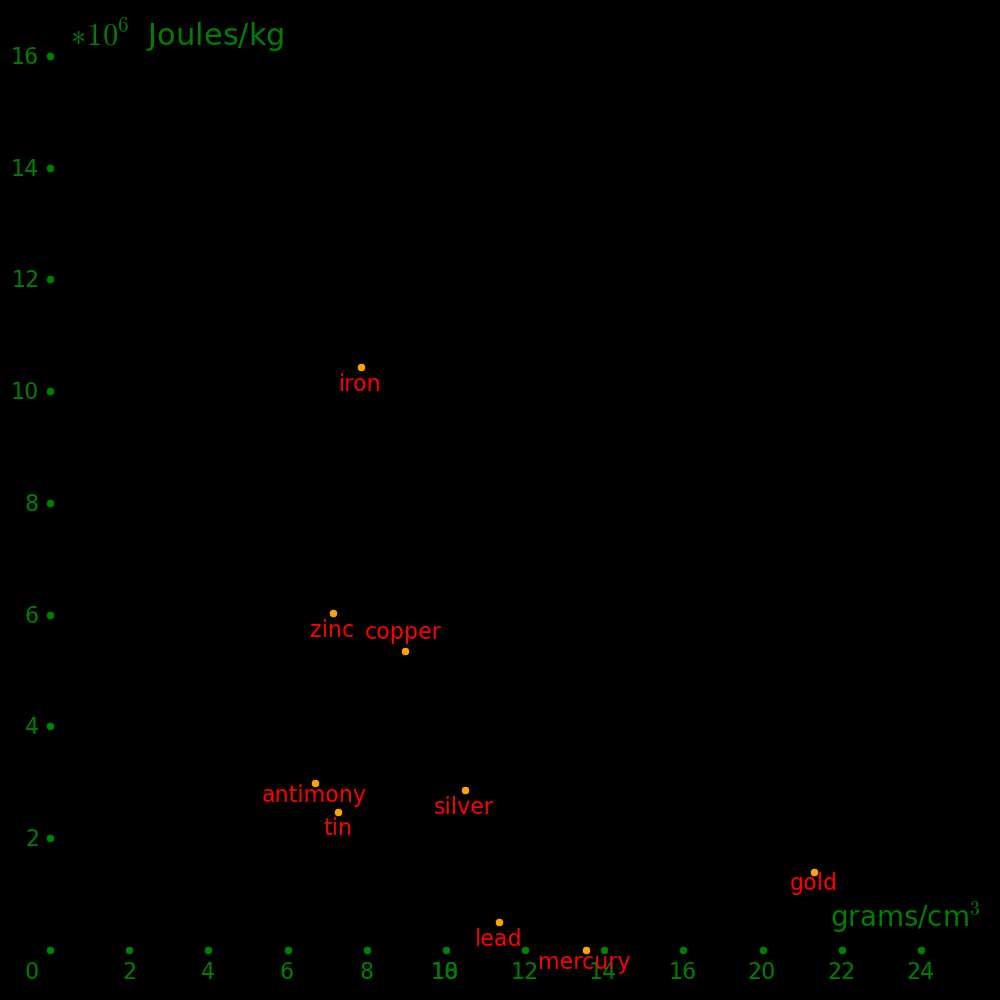
Horizontal axis: Density Vertical axis: Shear modulus / Density (Strength-to-weight ratio)
 |
|---|
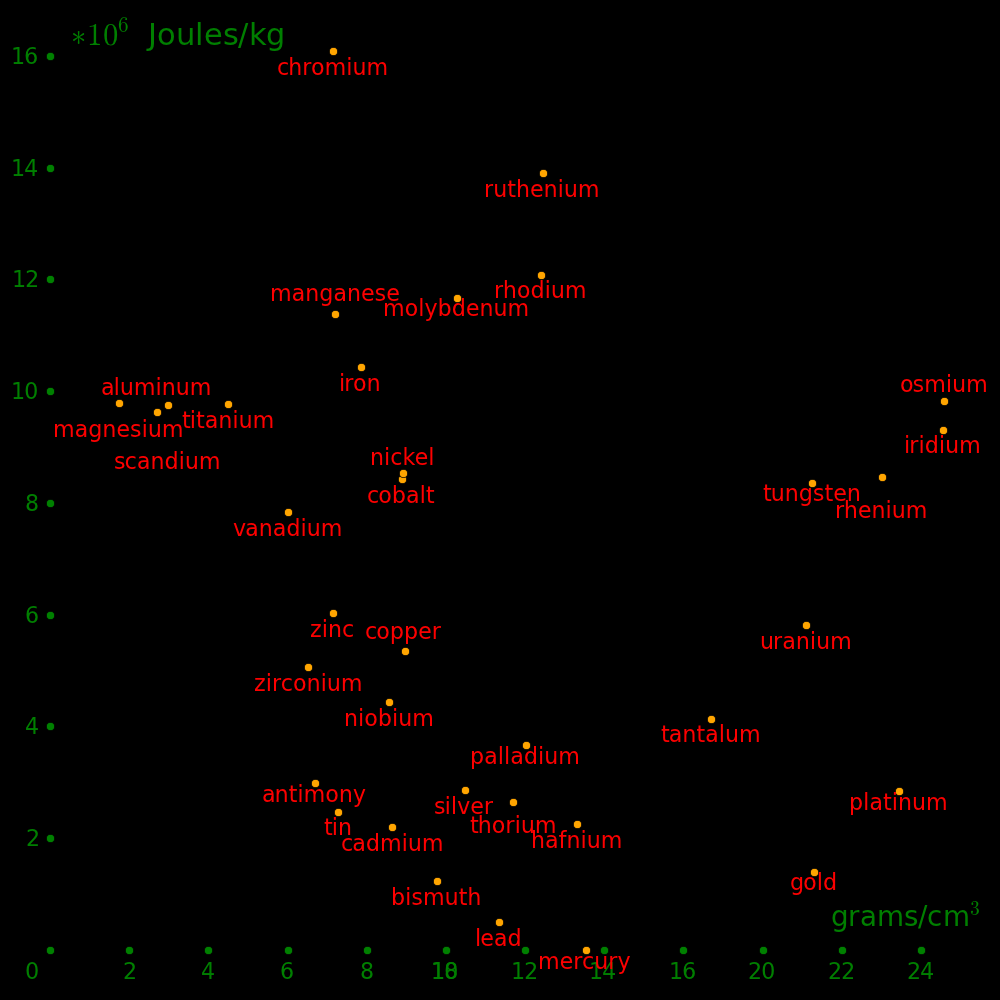
Horizontal axis: Density Vertical axis: Shear modulus / Density (Strength-to-weight ratio)Metals with a strength-to-weight ratio less than lead are not included, except for mercury.
 |
|---|
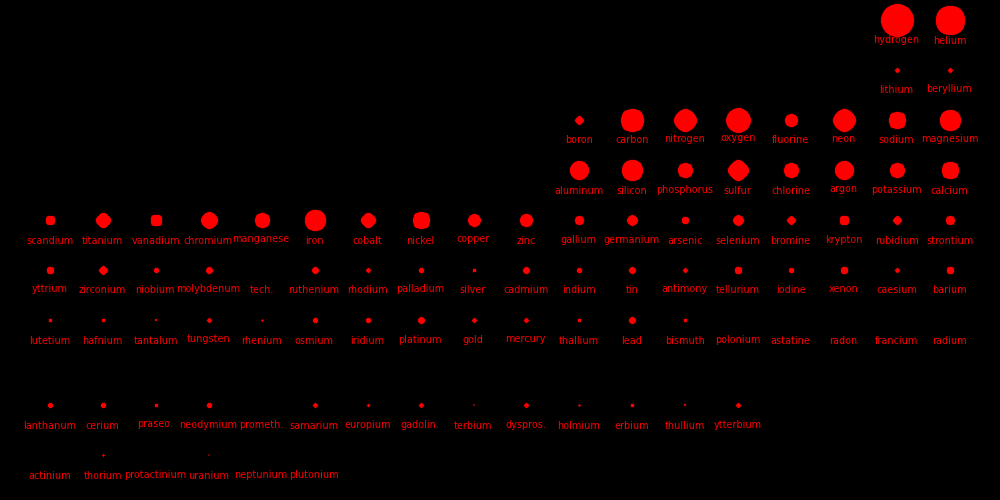
Dot size = log(SolarAbundance)Elements with a dot size of zero have no stable isotope.
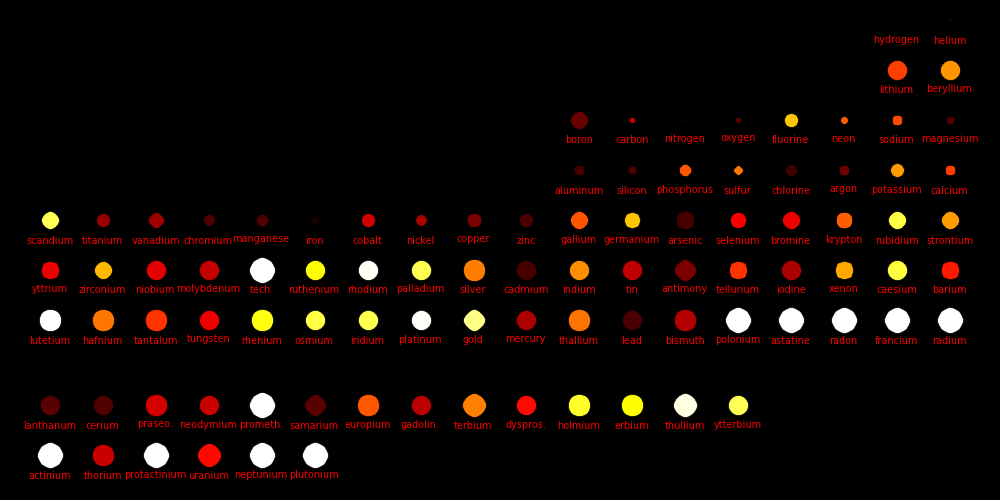 |
|---|
Color = Price per kilogram Dot size = -log(SolarAbundance) The smaller the dot, the more abundant the element.Price data
 |
|---|
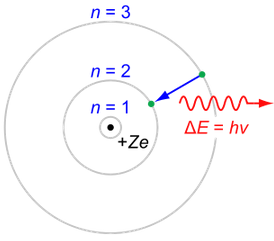
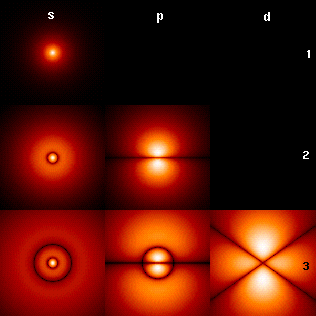
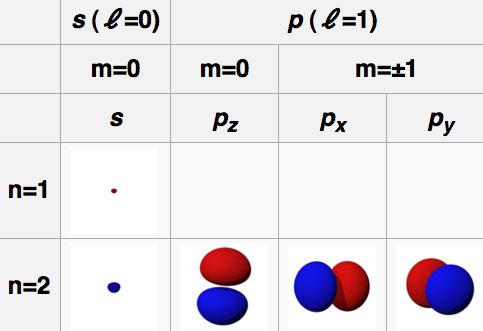
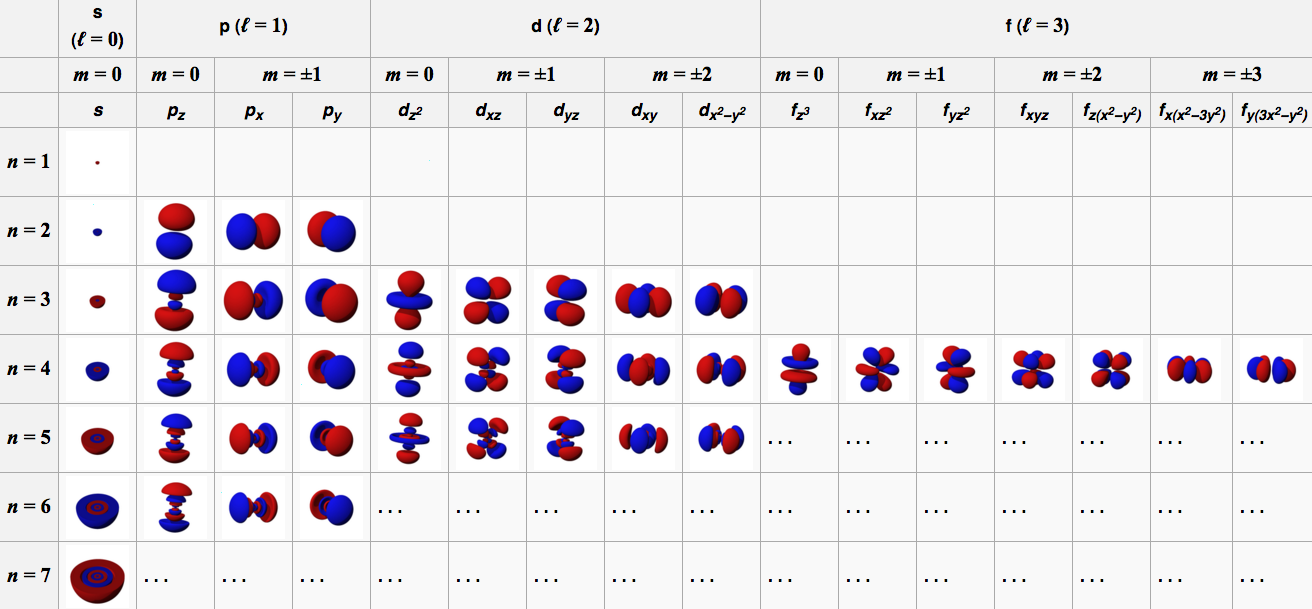
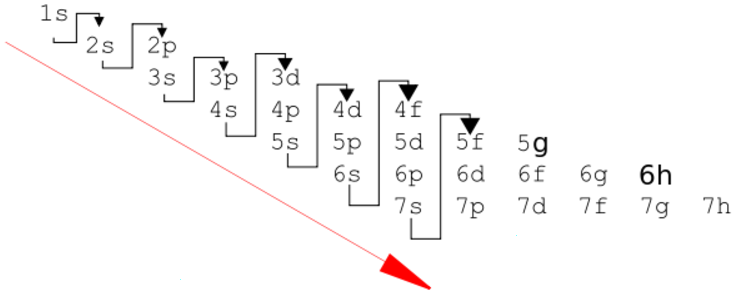
The "de Broglie wavelength" of a particle is
Particle momentum = Q Planck constant = h = 6.62*10^-34 Joule seconds Particle wavelength = W = h/Q (de Broglie formula)The Bohr hypothesis states that for an electron orbiting a proton, the number of electron wavelengths is an integer. This sets the characteristic size of a hydrogen atom.
Orbit circumference = C = N W where N is a positive integer N Orbital 1 S 2 P 3 D 4 F Electron mass = m = 9.11*10-31 kg Electron velocity = V Electron momentum = Q = m V Electron charge = e = -1.60*10-19 Coulombs Coulomb constant = K = 9.0*109 Newtons meters / Coulombs2 Electric force = Fe = K e2 / R2 Centripetal force = Fc = M V2 / R Orbit radius = R = N h2 / (4 π2 K e2 m) = N * 5.29e-11 meters Electron energy = E = - .5 K e2 / (R N2) = N-2 2.18e-18 Joules = N-2 13.6 electron Volts (Ionization energy)For an electron on a circular orbit,
Fe = Fc
 |
 |
|---|---|
 |
|---|
 |
 |
|---|---|
 |
|---|
 |
|---|
 |
|---|