




 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
 |
 |
 |
||
|---|---|---|---|---|---|
 |
 |
|---|---|
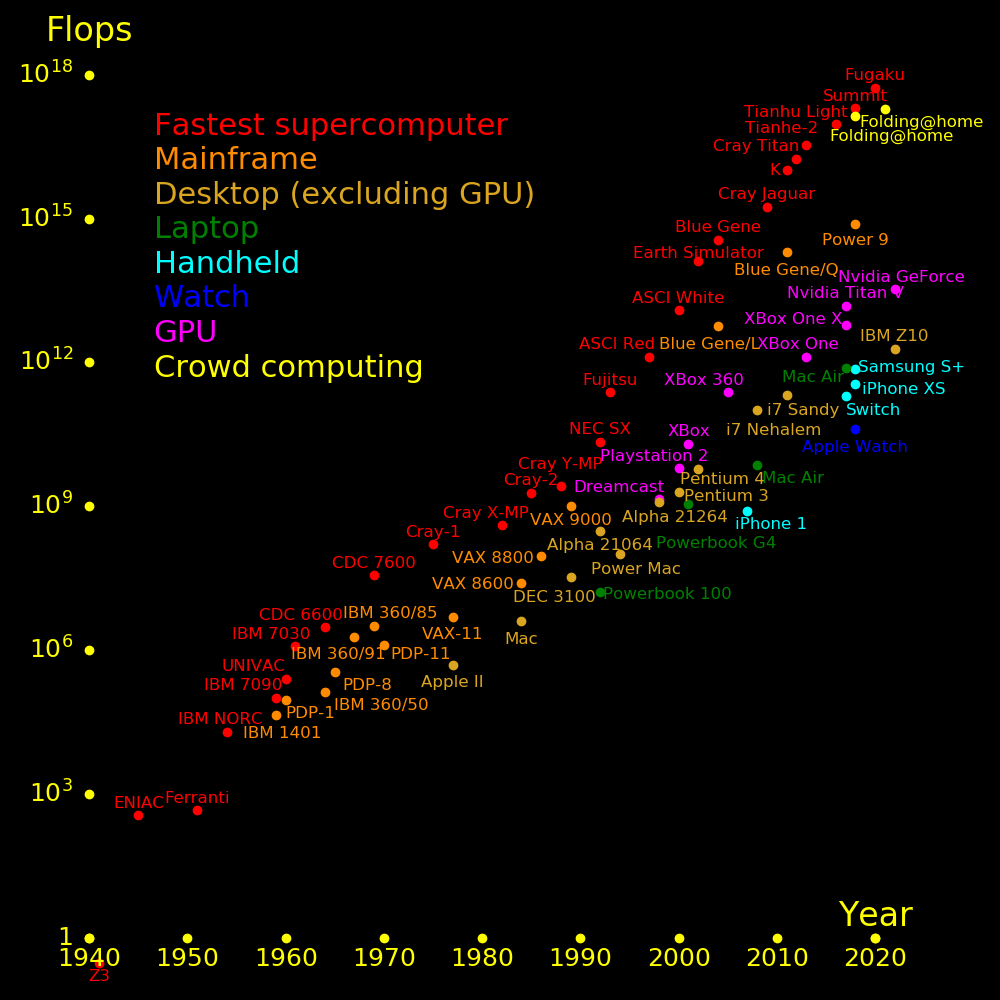
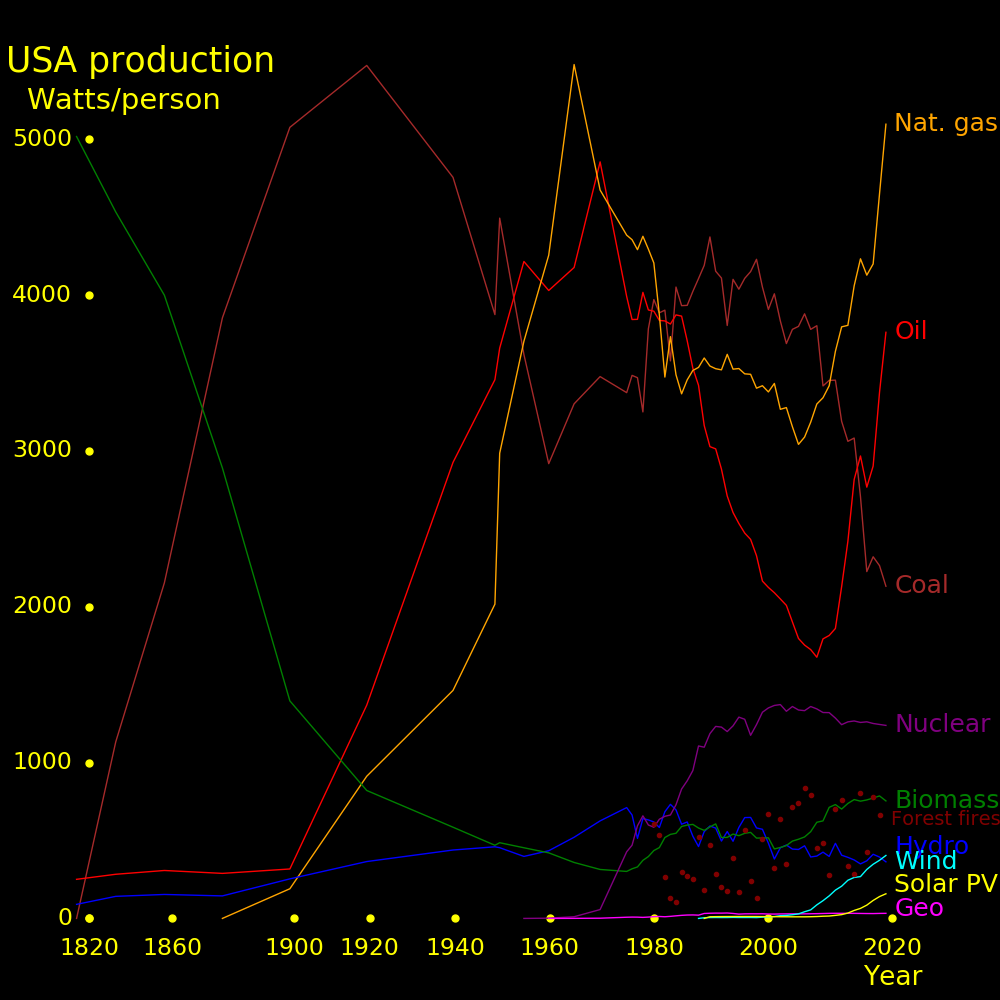
The world's fastest supercomputer is 10 times faster than a brain in terms of operations per second. A gamestation is 2500 times slower than a brain.
A brain is 3 orders of magnitude better than a computer for speed per power.
 |
|---|
A brain's clock time is the time of a chemical synapse (2 milliseconds) plus the signal crossing time across the brain (2 milliseconds), for a total of 4 milliseconds, or 250 Hertz.
Speed Clock Elements Power Speed/Power Fuel/Year Fuel
Ops/sec Hertz Watts Ops/Joule $/year
Brain 2⋅1016 250 1014 20 1014 3000 Food
Supercomputer 2⋅1017 4⋅109 25000000 700000 3⋅1011 600000 Electricity
Gamestation 8⋅1012 4⋅109 1000 1000 3⋅1011 1000 Electricity
For a computer, "elements" is the number of floating point units, and for a brain, it's the number of synapses.
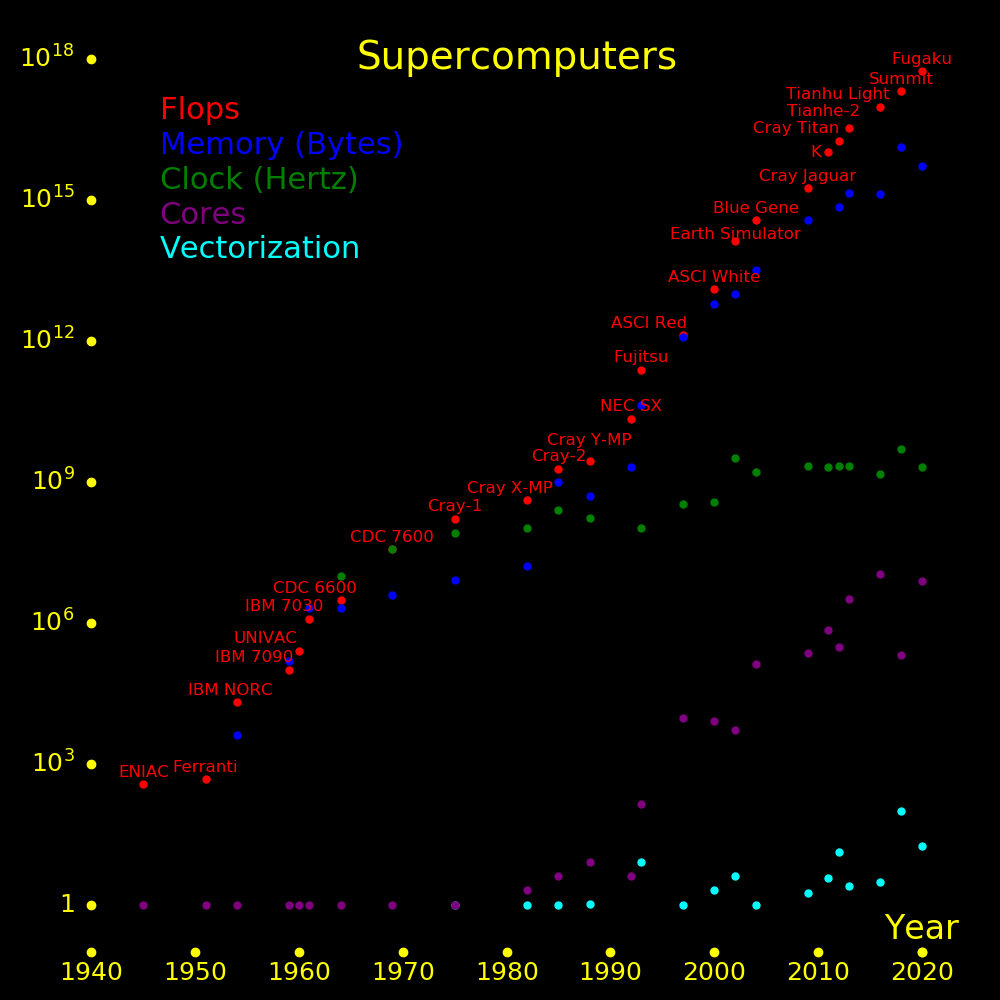
Clock speed topped out. The way forward is parallelization and vectorization.
 |
|---|
Computation speed is measured in GFlops (Giga Floating point operations per second). A floating point operation (Flop) is an add or a multiply.
A "core" is an independent floating point unit. Different cores can do different computations.
A core produces an add and a multiply once every clock cycle, hence it produces 2 floating point operations per cycle.
A core can be "vectorized" (a GPU), which means that it does many adds and multiples simultaneously. For vectorization, each element in the vector has to do the same computation. Gaming hardware is heavily vectorized. Computers became parallelized in the 1990s and vectorized in the 2010s.
The speed of a computer is
Supercomputer speed = S = 2FCV Clock frequency = F Cores = C Independent CPUs Vectorization = V Number of GPU vectors per core
Supercomputing is driven by Flops/$ and mobile computing is driven by Flops/Watt. For 2023,
Speed per dollar, CPU = 2 GFlop/$ Speed per dollar, GPU = 40 GFlop/$ Memory, RAM = .4 GByte/$ Memory, solid state = 7 GByte/$ Memory, disk = 33 GByte/$ Speed per power, GPU = 300 GFlop/Watt Battery energy per mass = .6 MJoule/kg Battery power per mass = 500 Watt/kg
Battery energy/mass and power/mass advance slowly. Computer speed/$ and speed/power advance rapidly.
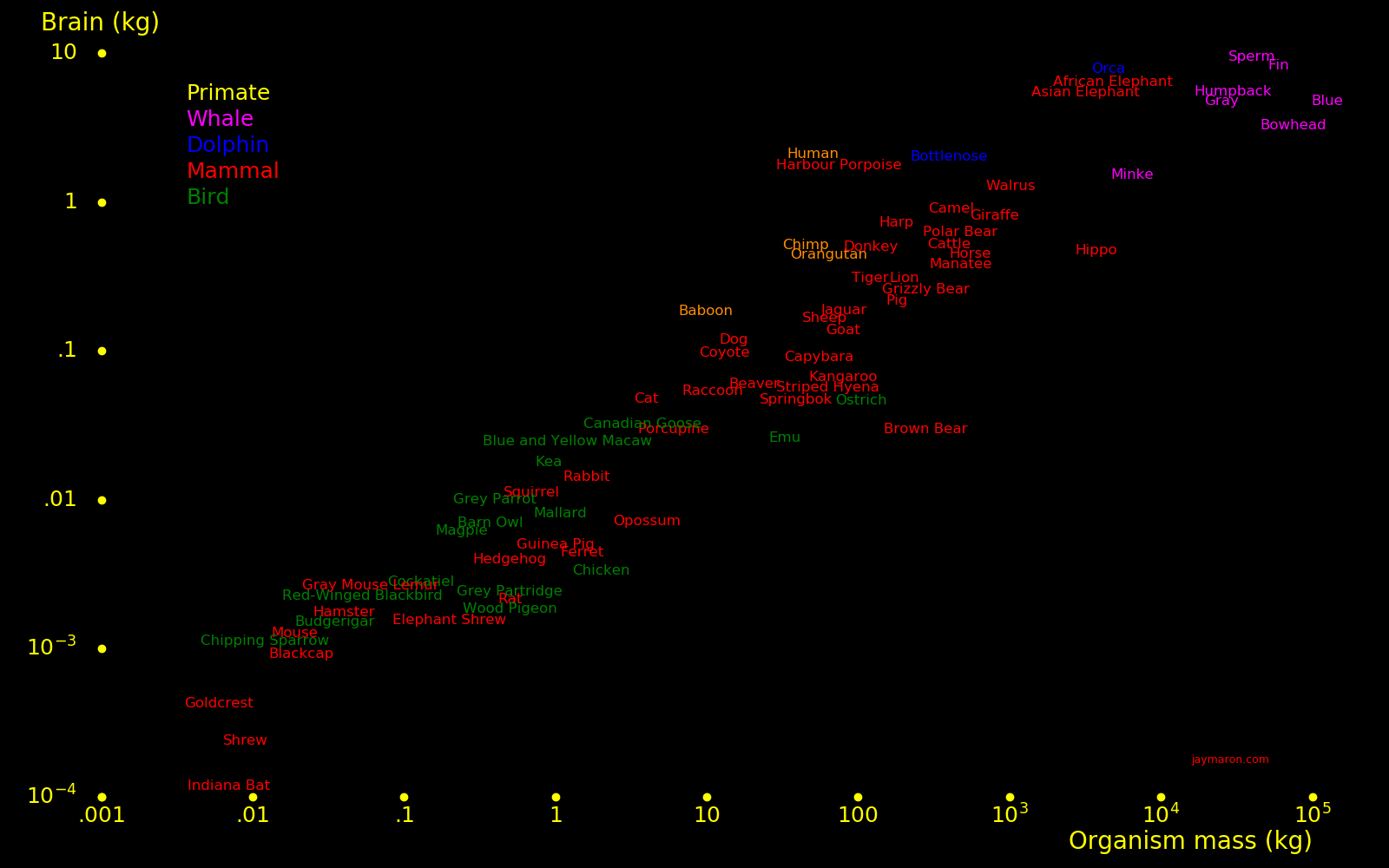
A sperm whale brain is 5 times larger than a human brain. It has 10 tons of audio organs and can sing louder than a jet engine.
 |
|---|
The table gives the year that a computer eclipsed the world champion.
Year World Champion Year the game was invented
Checkers 1990 Alexei Chizhov 1243
Scrabble 2000 1938 At the time, there was no consensus world #1 player
Chess 2006 Vladimir Kramnik 650
Go 2016 Lee Sedol -400
Shogi 2017 Yoshiharu Habu 1058 Japanese chess
The chess player Edward Lasker said:
"While the Baroque rules of Chess could only have been created by humans, the rules of Go are so elegant, organic, and rigorously logical that if intelligent life forms exist elsewhere in the universe, they almost certainly play Go."
The rules of chess are an example of "fine tuning" and there are lots of free parameters (the moves allowed by each piece).
 |
|---|
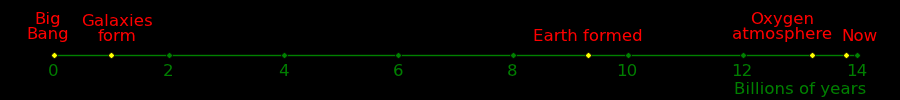
Most stars in the galaxy were formed before the sun. If alien exist, they likely have a head start on us by billions of years.
The sun is 23000 light years from the galactic center. A fusion drive can move at 1/10 the speed of light and can cross the galaxy in 100,000 years. If aliens want to be here, they would be.
Intelligent life requires an oxygen atmosphere because aerobic respiration yields vastly more energy than anaerobic respiration. A human brain uses 20 Watts and this would be hard to power anaerobically.
There are likely few planets that achieve an oxygen atmosphere. You need abundant water and photosynthesis. You also need to not overdo it with water and be a waterworld. You need both continents and oceans.
In the Age of Dinosaurs, there was much more biomass than today, and the atmospheric oxygen fraction was 38%. This enabled 80-kg organisms to fly.
Aerobic respiration yields more energy than anaerobic respiration.
Glucose + Oxygen → 30 ATP of energy Aerobic Glucose + Sulfur → 2 ATP of energy Anaerobic
 |
 |
 |
 |
|---|---|---|---|
 |
 |
 |
 |
|---|---|---|---|
Elements of the tetrapod design include:
A spine
A skull
A ribcage
Four limbs
One bone in the upper limbs, enabling shoulders and hips to be universal joints.
Two bones in the lower limbs, meaning that elbows and knees are linear joints.
The reason for 2 bones in the lower limbs is to control torque in hands and feet.
The diaphragm works with the ribcage and gut to generate suction to take in air.
Humans have the most complex wrists and hands in the animal kingdom. Only humans can throw rocks accurately.
Tetrapods include mammals, birds, lizards, and amphibians. Fish are not tetrapods. Whales and dolphins are tetrapods. Snakes once had limbs and many snakes lost them.
The tetrapod design emerged 370 million years ago and the first land animals emerged 350 million years ago.
Bruce Lee: There is only one type of body, 2 arms, 2 legs, etc that make up the
human body. Therefore, there can only be one style of fighting. If the other
guy had 4 arms and 2 legs, there might have to be a different one.
 |
|---|
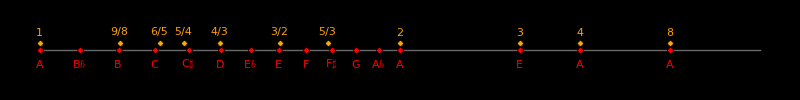
The 12-tone system is fortuitous. For a 12-tone octave, ratio tuning and logarithmic tuning give nearly the same notes. Aliens will use the 12-tone scale.
The 7 harmonic scales and the 7 melodic scales are decided naturally, by mathematics. Aliens will use these scales.
 |
|---|
A civilization can be ranked by its means for generating power. Earth civilization uses 20 Terawatts.
Civilization Level Year Pre-fire 1 Ancient Human power. 100 Watts/person Fire 2 -400000 Elastic power (bow & arrow) 3 -60000 Domestic animal power 4 -4000 One horsepower = 746 Watts Wind power 5 -3000 A large ship from Ancient Greece is 10000 Watts of wind power Hydro power 6 -400 Explosion power 7 850 Gunpowder Heat engine 8 1714 Sports car = 300 kWatt Nuclear fission 9 1945 Earth has 1025 Joules of uranium and thorium Asteroid power 10 Future The asteroid belt has 1030 Joules of kinetic energy Photovoltaic cells around sun 11 Future Sun = 4⋅1026 Watts Photovoltaic cells, big star 12 Future eta Carinae = 2⋅1033 Watts, distance = 7500 light years Galactic center 13 Future Milky Way center = 1036 Watts. Thousands of stars with more than 1 million solar luminosities each Galaxy 14 Future Milky Way = 1038 Watts. Virgo central galaxy = 1040 Watts Supermassive black hole 15 Future A quasar is up to 1041 Watts
Science fiction often invokes exotic matter. The history of materials is:
 |
|---|
The history of exotic matter is:
Exist Found
Gold Yes Ancient Naturally-occuring. Smelting was discovered in 6000 BCE.
Silver Yes Ancient Naturally-occuring. Smelting was discovered in 4000 BCE.
Copper Yes -5000
Tin Yes -3200 Bronze = Copper + Tin. Bronze is stronger than copper
Iron Yes -1200 Iron is stronger than copper
Cobalt Yes 1735 First metal discovered since ancient times
Chromium Yes 1797 Lighter, stronger, and harder than iron. Candidate for mithril and Valyrian steel
Tungsten Yes 1783 Much stronger than iron. Candidate for adamantium, vibranium, and duranium
Magnesium Yes 1808 First metal produced by electrolysis
Charged matter Yes -600 Static electricity
Magnet Yes -500
Antimatter Yes 1932 Antiprotons, antielectrons, antineutrons, etc.
Anticarbon Yes No Requires an antistar
Antiuranium Yes No Requires an antisupernova
Antimachines Yes No Require antilife
Photon, Infrared Yes 1800
Photon, UV Yes 1801
Photon, X-ray Yes 1896 Discovered using high voltage
Photon, Gamma Yes 1900 Discovered as gamma decay
Electron Yes 1897
Proton Yes 1919
Neutron Yes 1932
Neutrino Yes 1956
Lepton: Muon Yes 1937
Lepton: Tau Yes 1975
Quark: Up Yes 1968
Quark: Down Yes 1968
Quark: Strange Yes 1968
Quark: Charm Yes 1968
Quark: Bottom Yes 1977
Quark: Top Yes 1995
Gluon Yes 1978 Boson that carries the strong force and holds quarks together
Weak matter Yes 1983 Weak bosons. W and Z
Higgs Yes 2012
Graviton Yes No Boson that carries the gravitational force
Dark matter Yes No DoesnO’t feel the strong or electric force
Strong matter Yes No Bosons that mediate the strong force, such as X and Y bosons
Feeble matter Likely No New weak bosons
Inflaton Yes No Cause of cosmic inflation
Dark energy Yes No Matter with negative pressure
Mirror matter Unlikely No Mirror versions of conventional particles
Magnetic monopole Likely No
Primordial black hole Unknown No
Anyon Unlikely No Particles with spin other than 0, 1/2, 1, 3/2, 2.
Tachyon Unlikely No Particles that are faster than light
Biquark (meson) Yes 1947 2 quarks
Tetraquark Yes Yes 2 mesons bound together
Pentaquark Yes Yes 5 quarks. 4 quarks and an antiquark
Glueonium Yes 2020 Composite particle consisting of gluons
Quark-gluon plasma Yes 2000
Suburanics Yes 1911 Polonium, radium, actinium, protactinium. Proton number from 84 to 89.
Transuranics Yes 1942 Plutonium through Fermium. Proton number from from 94 to 100.
Neutronic matter Yes Yes Radioisotopes that are neutron-rich.
Protonic matter Yes Yes Radioisotopes that are proton-rich. Most medical isotopes are protonic
Superheavy matter Likely No Nuclei in the hypothetical "island of stability", with atomic number around 112
A 2nd island of stability exists around atomic number 122
Up-down quark matter Unknown No Nuclei with more than 300 nucleons may transition to udQM and be long-term stable
Strange matter Unlikely No Superheavy nuclei with strange quarks
Aether Unlikely No Carrier of photons
Negative energy Unlikely No
Synthetic sapphire Yes 1902
Synthetic diamond Yes 1954
Amorphous alloy Yes 1980 Light and strong
Buckyball Yes 1984
Carbon nanotube Yes 1993
Graphene Yes 2004
Superconductor Yes 1911
Room-T superconduct Unknown No
The following questions are up for grabs.
Does a superconductor exist at room-temperature and zero pressure?
Does a gamma laser exist that uses nuclear transitions?
Does the nuclear "island of stability" exist?
Do superheavy nuclei exist?
Each technology has a current state-of-the-art and a future maximum. Sometimes the future maximum can be calculated and sometimes not. The most important physical limits that have the potential to improve are:
Current Future Unit Current state Future state
limit limit of art of art
Superconducting max temperature 134 ? Kelvin HgBa2Ca2Cu3O8
Transistor size 80 ? nm
Permittivity (relative) 250000 ? Dimensionless CaCuTiO3
Permeability (relative) 1000000 ? Dimensionless Metglass 2714A
Shear strength/density 860 3000 MJoule/kg Carbon fiber Diamond nanobeams that are isotopically-pure
Battery, radioisotope, efficiency .1 .15 Dimensionless
Temperature low 38 ? picoKelvin Laser cooling
Infrared detector limit 20 ? microns Gallium arsenide
Particle energy 6.5 ? TeV Large Hadron Collider
Particle accelerator force 100 ? MeV/meter Dual-beam system
Time precision 10-16 ? Dimensionless Caesium clock
Length precision 10-21 ? Dimensionless LIGO
Technologies that are nearly maxed out are:
Current Future Unit Current limit Future limit
limit limit
Melt temperature 4232 4370 Kelvin HfTaC HfCN
Battery, energy/mass, Li-ion cobalt .8 1 MJoule/kg
Battery, energy/mass, Li-ion sulfur 1 1.8 MJoule/kg
Tensile strength 130 GPascal Graphene
Tensile strength/mass 130 MJoule/kg Graphene
Magnetic field, permanent magnet 1.25 Tesla Neodymium magnet
Magnetic field, continuous, supercon 32 Tesla
Magnetic field, continuous, resist 38 Tesla
Magnetic field, superconduct crit 55 Tesla MgB2
Does strange matter exist?
Does mirror matter exist?
Do primordial black holes exist?
Do magnetic monopoles exist?
Do cosmic defects exist?
Does time travel exist?
Do we live in a false vacuum?
 |
|---|
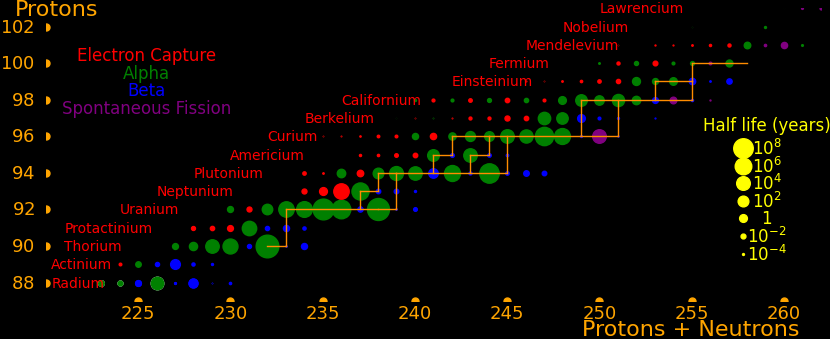
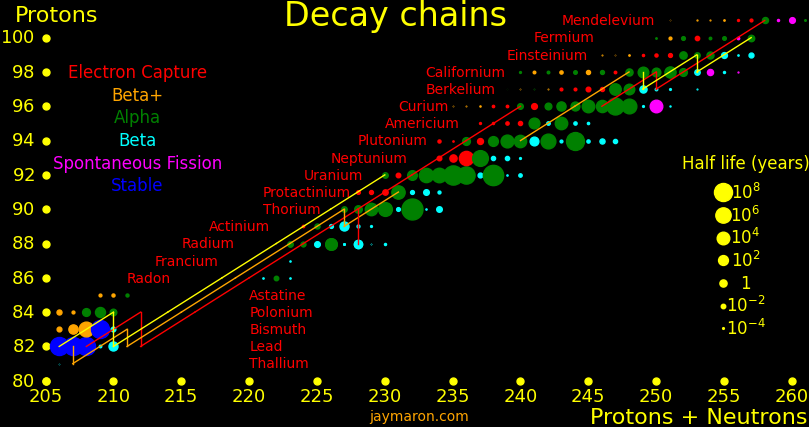
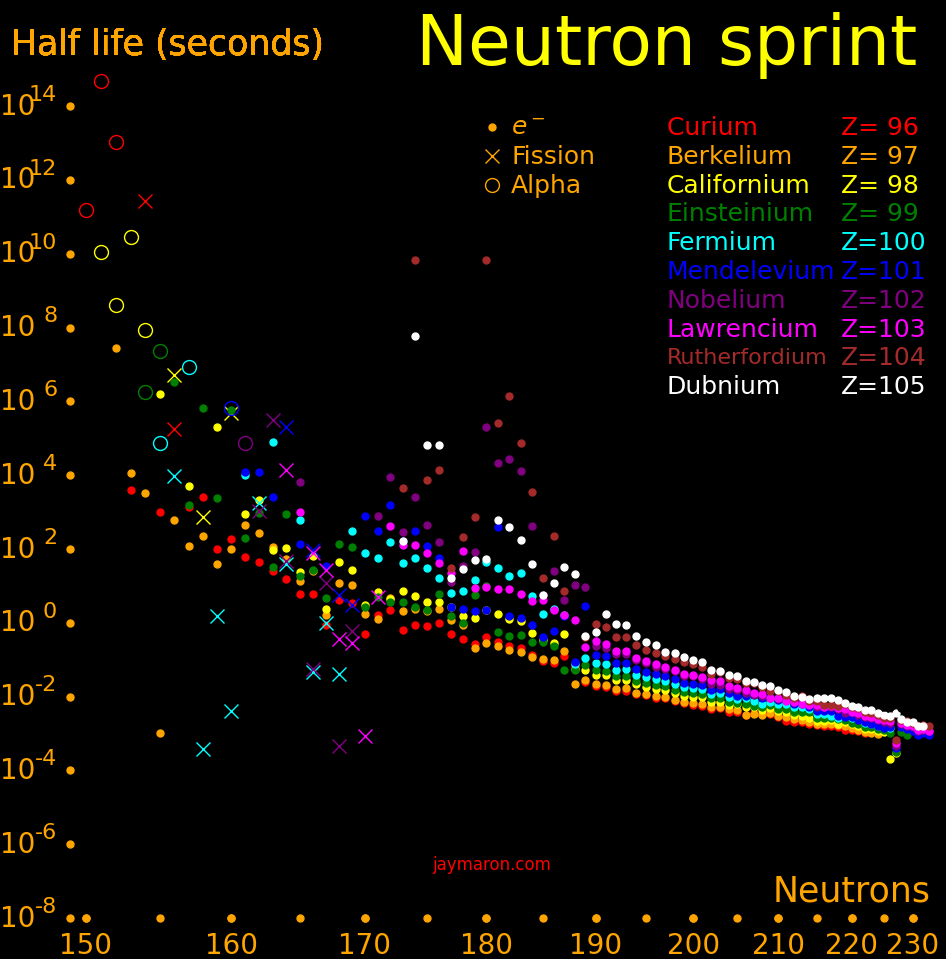
Many exotic isotopes are actinides, the elements surrounding uranium. The orange lines show which isotopes can be made with neutron transmutation.
 |
|---|
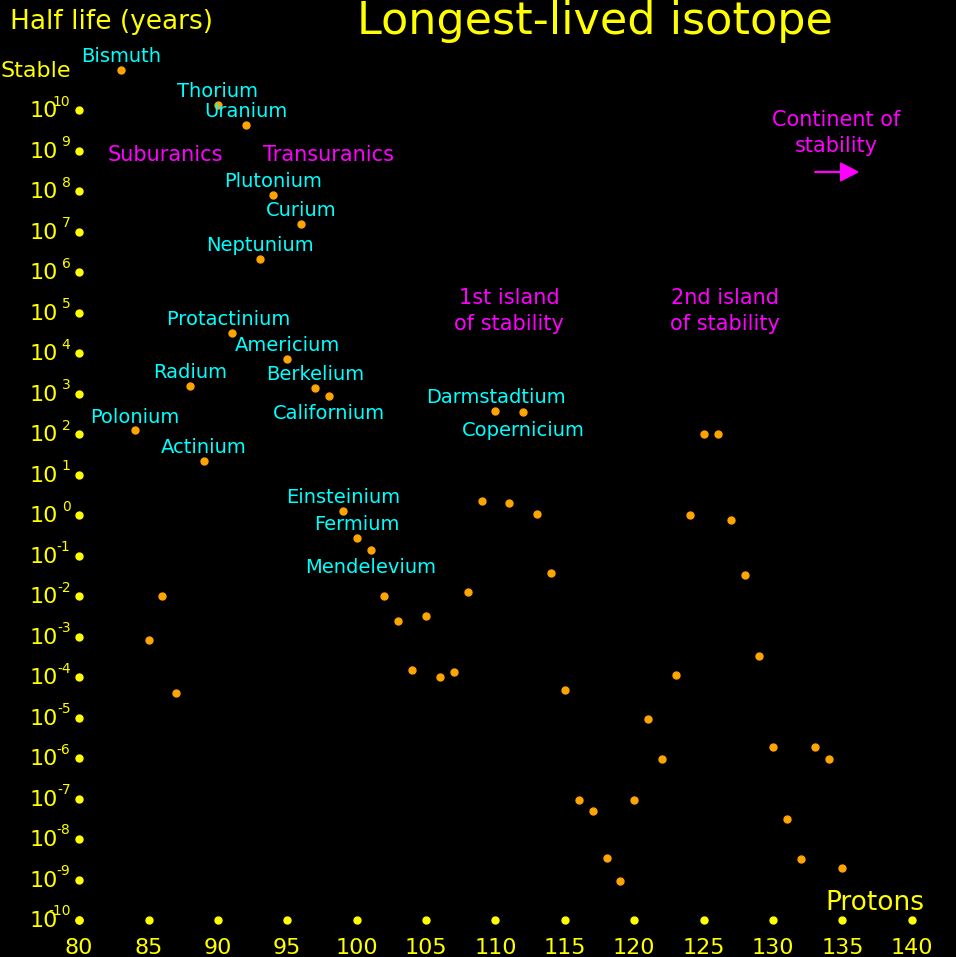
There is a hypothetical "island of stability" around atomic number 112 where nuclei may be long-term stable.
Experiments can only measure the longest-lived isotope up to a proton number of 105, and beyond that we plot theory. Theoretical half lives are uncertain by an order of magnitude.
It's possible that for large nucleon number, larger than around 300, that the nucleus transitions to a lower-energy state, called "Up down quark matter", or "udQM". The existence of udQM is unresolved. Theory is uncertain, and it hasn't been experimentally produced. The largest nucleus that's been produced is oganesson-294, with 118 protons and 294 nucleons. It shows no sign of udQM, so if udQM exists, it's beyond oganesson.
If udQM nuclei exist, they could potentially be long-term stable. They don't fission because it would take the nucleus to a higher-energy state. They decay by alpha until they're too light to be udQM, at which point they fission.
If udQM nuclei exist, then there may be exist long-lived elements from Z=140 to way beyond. These are "continental elements".
The largest nucleus that standard nuclei can make has Z=140. Nuclei larger than this fission with a short half life. The only way that nuclei with Z>140 can exist is if udQM exists.
 |
|---|
If continental elements exist, we can guess their properties by extrapolating from homolog elements. Homologs are elements in the same column of the periodic table.
Continental Homolog Extrapolated properties element element 118 Radon Noble gas 119 Caesium Good for atom traps 121-153 Lanthanides Exotic properties for solid state physics 154 High melting point, . High hardness 155 Tantalum High melting point. High hardness. STaC melt 4600 Kelvin 156 Tungsten High melting point. High hardness. SW melt 4800 Kelvin 157 Rhenium 158 Osmium Density = 40 g/cm3 159 Iridium 160 Platinum Catalyst 161 Gold 162 Mercury 168 Radon
For each kind of object, the table gives the nearest one and also superlative examples of the object.
Distance Mass Radius Luminosity
light year Sun=1 Sun=1 Sun=1
Star Proxima Centauri 4.24 .123 .141 .0017 Nearest
Star Alpha Centauri A 4.36 1.10 1.23 1.52 Nearest sun-sized star
Star Sirius A 8.7 2.063 1.71 25.4
Star Regulus 77 3.8 4.35 316
Star Dschubba 136 13 6.7 38000
Star Naos 1080 56 20 813000
Star Eta Carinae 7500 100 240 4600000 Among the most luminous stars
Red giant Arcturus 37 1.1 26 170 Nearest
Red giant Betelgeuse 700 18 764 126000
White dwarf Sirius B 8.7 1.02 .0084 .056 Nearest
Neutron star RX-J1856 400 .9 Nearest
Pulsar J0108 424 Nearest
Pulsar Vela pulsar 959 89 Hertz
Pulsar PSR J1614-2230 1200 1.91 317 Hertz
Pulsar Crab pulsar 6500 .9 30 Hertz. Brightest gamma source. Gammas up to 10 TeV. 1.6 mllion Kelvin
Pulsar PSR J1748-2446ad 18000 716 Hertz. Highest spin rate
Magnetar AXP 1E 1048.1-5937 9000 Nearest
Magnetar SGR1806-20 42000 Strongest magnetic field at 1011 Tesla
Supernova SN1250 700 Nearest
Star-forming cloud Corona Nebula 430 Nearest
Star-forming cloud Orion Nebula 1344
Black hole Gaia BH1 1560 9.6 Nearest
Black hole Cygnus X-1 7300 30
Black hole Milky Way center 25600 4200000 .019 Nearest supermassive black hole
Black hole Andromeda 2300000 200000000 .85
Black hole Sombrero galaxy 31100000 1000000000
Black hole Virgo A 53500000 6500000000 Virgo Cluster
Black hole NGC1600 149000000 17000000000 Not in a cluster of galaxies
Black hole NGC6166 490000000 28000000000 Abell 2199
Black hole Holmberg 15A 700000000 40000000000 Abell 85
Black hole 4C+37.11 750000000 15000000000 2 holes separated by 24 light years. Total mass given
Black hole MS0735.6 2600000000 51000000000
Black hole Phoenix A 8610000000 100000000000 424000 Most massive. 3e39 Watts
Galaxy Andromeda 2300000 Nearest galaxy that's the size of the Milky Way
Galaxy Virgo central 53000000 Biggest galaxy in the nearest cluster of galaxies
Active galaxy Centaurus A 12000000 55000000
Active galaxy Messier 81 11700000 70000000
Quasar Markarian231 581000000 4000000 Nearest
Quasar 3C 273 2440000000 886000000 4e12 Brightest
Quasar Ton 618 10800000000 50000000000 e14 4e40 Watts
Quasar MSS J215728 12500000000 34000000000 7e14 Most luminous. 2.6⋅1041 Watts
 |
|---|
Kirk's interpretation of the Prime Directive is often overly-liberal. You could say that he considers it to be the "Prime Suggestion". If aliens exist then it's reasonable to suppose that they may have something like a Prime Directive, because as yet, no evidence for them has been found.
 |
|---|
One could imagine aliens creating a "doomsday machine" with orders to replicate itself and travel to every star in the galaxy, and upon arriving, exterminate all life present. If this had happened then we would not be here now. The non-existence of a doomsday machine is one of the few solid assertions that we can make about aliens.
That being said, it may be that there is a doomsday machine waiting to exterminate
Earth once we reach a specified technological level.
 |
 |
 |
|---|---|---|
 |
 |
 |
 |
 |
 |
|---|---|---|---|---|---|
 |
 |
 |
|
|---|---|---|---|
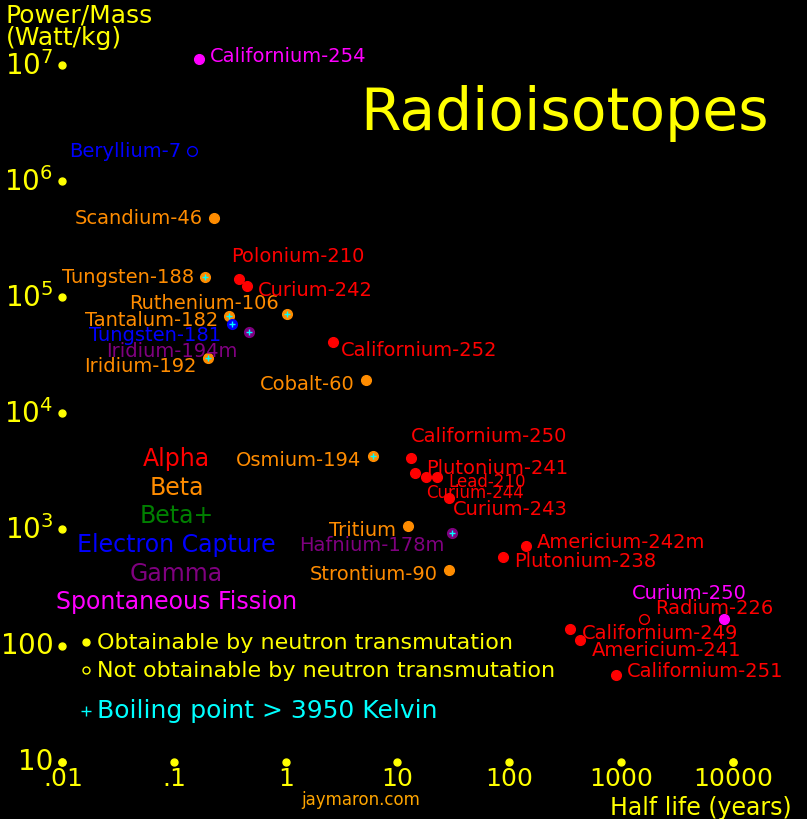
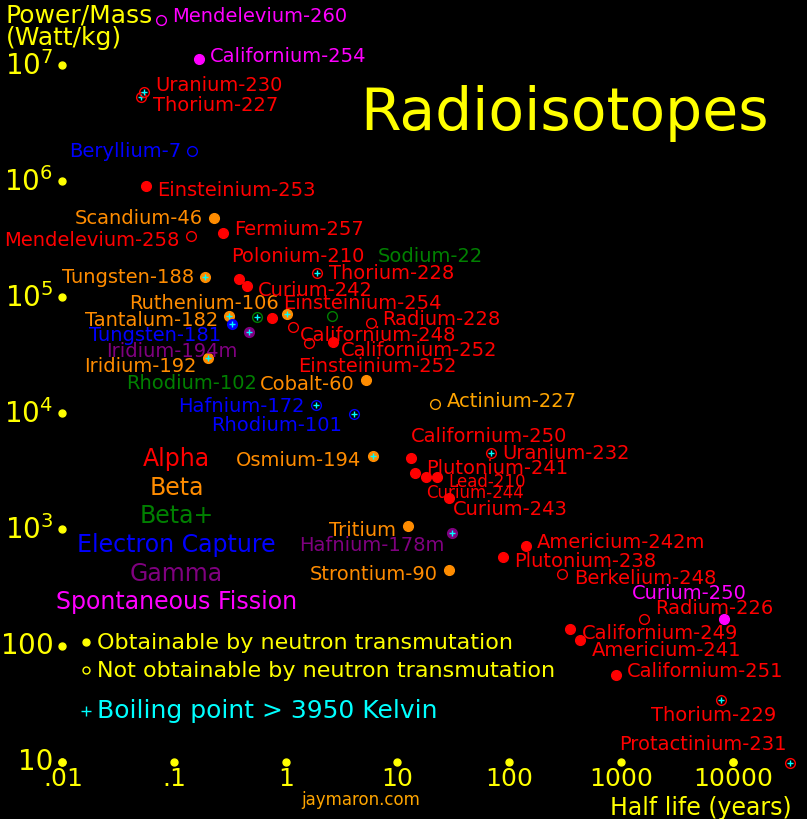
Radioisotopes have more energy per mass than gasoline by a factor of 1 million. They can power an aircraft for 50 years and they power the Voyager spacecraft. They can make a fission bomb the size of a softball. Iron Man gadgets are possible with them. Important isotopes include:
Half life (year) Use
Tritium 12.3 Fusion fuel
Helium-3 Stable Fusion fuel. Dilution refrigerator. Fission afterburner rocket
Uranium-235 704000000 Fission fuel. Fission bomb
Plutonium-239 14100 Fission fuel. Fission bomb
Thulium-170 .352 Power
Polonium-210 .379 Power. Alpha rocket
Thorium-228 1.91 Power. Alpha rocket
Thulium-171 1.91 Power. Safe around humans because it requires low radiation shielding
Caesium-134 2.06 Power
Cobalt-60 5.27 Power
Europium-152 13.5 Power
Strontium-90 28.9 Power
Uranium-232 68.9 Power. Alpha rocket
Plutonium-238 87.7 Power. Alpha rocket
Radium-226 1599 Power. Breeder for Thorium-228 and Uranium-232
Lithium-6 Stable Fission afterburner rocket. Fusion bomb
Boron-10 Stable Fission afterburner rocket. Cancer treatment
Beryllium-7 .146 Fission afterburner rocket
Sodium-22 2.602 Fission afterburner rocket
Americium-242m 141 Fission afterburner rocket
Californium-251 900 Fission afterburner rocket. Compact fission bomb. Large neutrons/fission
Californium-252 2.65 Spontaneous fission. Compact fission bomb. Large neutrons/fission
Curium-250 8300 Spontaneous fission
Carbon-12 Stable Isotopically-pure diamonds, nanotubes, and graphene
Carbon-14 5700 Low neutron capture cross section
Iridium-192 .202 Power at high temperature
Tantalum-182 .313 Power at high temperature
Tungsten-181 .332 Power at high temperature
Osmium-194 6.02 Power at high temperature
 |
 |
|---|---|
Isotope radioactivity can make electricity. The Voyager spacecraft are powered by plutonium-238.
 |
|---|
The isotopes with high power/mass are:
Half life Heat Decay Decay Obtainable by
year Watt/kg MeV neutron transmutation
Californium-254 .166 11200000 Fission 207 *
Iridium-192 .202 29942 β 1.460 *
Scandium-46 .229 485000 β 2.366 *
Thulium-170 .352 33150 β .968 *
Thulium-171 1.91 606 β .0965 *
Caesium-134 2.06 15300 β 2.059 *
Californium-252 2.64 41400 α or Fission 12.33 * α 96.9% (6.12 Mev). Fission 3.09% (207 MeV)
Cobalt-60 5.27 19300 β 2.824 *
Europium-154 8.59 3030 β 1.968 *
Tritium 12.33 315 β .0186 *
Europium-152 13.5 1821 β & EC 1.86 *
Strontium-90 28.9 2234 β, &beta 2.826 *
Caesium-137 30.1 583 β 1.176 *
Plutonium-238 87.7 578 α 5.59 *
Americium-242m 141 725 2α 12.33 *
Silicon-32 153 1159 β, β 1.92 *
Iridium-192m 241 72 IT, β 1.615 *
Curium-250 8300 170 Fission 148 * Fission 74%, Alpha 18%, Beta 8%
Beryllium-7 .146 1822000 EC .547
Hafnium-172 1.87 11700 EC 1.835
Hafnium-172 1.87 11700 EC 1.835
Sodium-22 2.6 68700 β+ or EC 2.842
Rhodium-101 4.07 9890 EC 1.980
Titanium-44 59.1 4318 EC, β+ 3.798
Thorium-227 .0512 9194000 5α+2β 36.14
Uranium-230 .0554 9280000 6α+2β
Thorium-228 1.912 235000 5α+2β 34.784
Radium-228 5.75 90660 5α+4β 40.198
Actinium-227 21.8 21600 5α+3β 36.18
Uranium-232 68.9 7545 6α+2β 40.79
Radium-226 1599 286 5α+4β 34.958
Thorium-229 7917 57.7 5α+3β 35.366
Protactinium-231 32600 16.2 6α+3β 41.33
Thorium-230 75380 6.78 6α+4β 39.728
 |
|---|
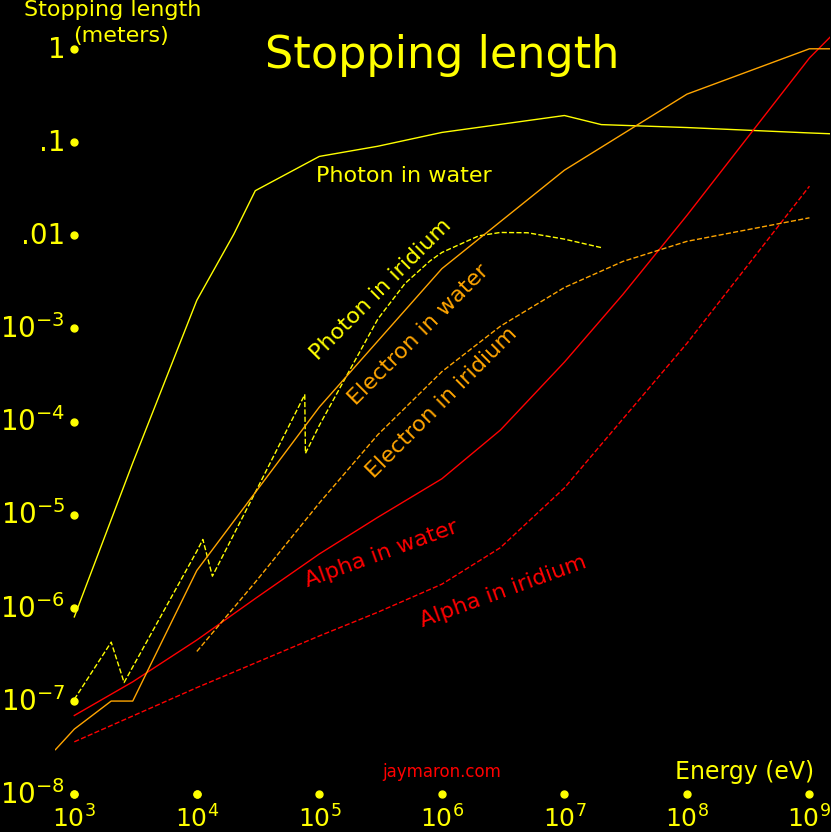
A battery that's around humans needs radiation shielding. Gammas are the most penetrating radiation, so what matters is the highest-energy gamma produced. Alpha and beta decay produce gammas by bremsstrahlung, and a charged particle can give all its energy to one gamma.
The isotopes with low-energy gammas are:
Half life Power/mass Decay Gamma Stopping Formation Obtainable by Decay
energy max length rate neutron
year Watt/kg MeV MeV mm barn*year transmutation
Nickel-63 100.1 5.52 .017 .017 .004 2.5 * β
Tritium 12.33 315 .0186 .0186 .005 71 * β
Rubidium-83 .236 .910 .0322 0 EC
Samarium-145 .931 .617 .061 .022 * EC
Tantalum-179 1.82 .110 .065 0 EC
Promethium-145 17.7 131 .164 .072 .022 * EC
Platinum-193 50 17.5 .057 .076 3.81 * EC
Thulium-171 1.91 606 .096 .096 .09 30.8 * β
Europium-155 4.76 705 .252 .147 312 * β
"Gamma" is the energy of the highest-energy gamma produced.
"Stopping length" is the stopping length of the gamma in iridium. The radiation shield should be at least 10 times thicker than the stopping length.
"Formation rate" indicates of how fast it can be produced in a nuclear reactor. See the "Hurdle" section below.
 |
 |
 |
 |
|---|---|---|---|
Isotopes exist with a critical mass smaller than plutonium-239. The smallest critical mass is californium-252 at 2.7 kg. The critical sphere diameter is 6.9 cm, the size of a tennis ball.
Half life Critical Critical Density Fission
mass diameter barn
year kg cm gram/cm3
Californium-252 2.64 2.73 6.9 15.1 33
Californium-251 900 5.46 8.5 15.1 4894
Californium-249 351 6 9 15.1 1666
Curium-247 15700000 7 9.9 13.5 82
Plutonium-239 14100 10 9.9 19.8 748
Uranium-233 159000 15 11 19.1 536
Uranium-235 704000000 52 17 19.1 538
Natural uranium has a critical mass of 800 with heavy water moderator, and 10000 kg with graphite moderator.
 |
|---|
An isotope that sponteously fissions is a neutron source. The easiest such isotope to make is californium-252. The isotopes with a large spontaneous fission rate are:
Fission Decay Neutrons Fission
half life Half life per fraction
years years fission
Mendelevium-260 .0895 .0761 .85
Californium-254 .166 .166 .997
Californium-252 85.7 2.65 3.73 .0309
Curium-250 11200 8300 3.31 .74
 |
|---|
A fission thermal rocket uses a fission reactor to heat hydrogen exhaust. The higher the temperature, the faster the exhaust. The temperature limit is of the order 4000 Kelvin because this is the highest temperature for which solids exist. The material with the highest melting point is hafnium carbide at 4201 Kelvin.
A fission reactor can alternately heat exhaust by generating neutrons, which trigger fission in the exhaust. The neutrons are generated as a pulse and the exhaust is pulsed. The likelihood for a neutron to trigger fission is quantified as a "cross section". The isotopes with the largest fission cross section are:
Half life Fission Energy Quality Neutron capture Obtainable by
year barn MeV output neutron transmutation
Americium-242m 141 7024 195 5640 Daughter nuclei + Neutrons *
Californium-251 900 4894 207 3940 Daughter nuclei + Neutrons *
Curium-245 8500 2161 198 1740 Daughter nuclei + Neutrons *
Plutonium-239 14100 748 189 590 Daughter nuclei + Neutrons *
Uranium-235 704000000 538 181 410 Daughter nuclei + Neutrons *
Beryllium-7 .146 56800 1.644 11670 Lithium-7 + Proton
Sodium-22 2.602 27490 4.14 4948 Neon-22 + Proton
Helium-3 Stable 5333 .764 1020 Tritium + Proton *
Boron-10 Stable 3835 2.34 820 Lithium-7 + Alpha *
Lithium-6 Stable 940 4.783 640 Alpha + Tritium *
Zirconium-88 .228 861000 8 77000 Zirconium-89 + Gammas
Gadolinium-157 Stable 259000 7.94 13020 Gadolinium-158 + Gammas *
Gadolinium Stable 49000 8 2500 Gadolinium-158 + Gammas * Natural composition
An isotope's quality as fission afterburner fuel is
Atomic mass number = M Dimensionless Fission energy = E MeV Fission cross section = A barns Afterburn quality = Q = AE/M
Many superisotopes are actinides. The orange line shows the isotopes can be produced by neutron capture.
 |
|---|
Neutron capture transmutes an isotope one space to the right and beta decay transmutes an isotope one space up. Isotopes on or to the right of orange lines can be made with neutron transmutation and isotopes to the left of the lines can't. Elements that are made by neutron-transmutation tend to be neutron-heavy (neutronic), and elements that can't be made by neutron-transmutation tend to be proton-heavy (protonic).
 |
|---|
Suburanics are the isotopes from radium to uranium. Many undergo 5 or 6 alpha decays, such as uranium-232. This is the red line in the plot.
 |
|---|
There is a hypothetical "island of stability" around atomic number 112 where isotopes may be long-lived. A second island may exist at atomic number 126.
Experiments can only measure the longest-lived isotope up to a proton number of 105, and beyond that we plot theory. Theoretical half lives are uncertain by an order of magnitude.
 |
|---|
It's possible that for large nucleon number, larger than around 300, that the nucleus transitions to a lower-energy state, called "Up down quark matter", or "udQM". The existence of udQM is unresolved. Theory is uncertain, and it hasn't been experimentally produced. The largest nucleus that's been produced is oganesson-294, with 118 protons and 294 nucleons. It shows no sign of udQM, so if udQM exists, it's beyond oganesson.
If udQM nuclei exist, they could potentially be long-lived. They don't fission because it would take the nucleus to a higher-energy state. They decay by alpha until they're too light to be udQM, at which point they fission.
If udQM nuclei exist, then there may exist long-lived elements from Z=140 to way beyond. These are "continental elements".
The largest nucleus that standard nuclear matter can make has Z=140. Larger nuclei fission instantly. The only way that nuclei with Z>140 can exist is if udQM exists.
Typical energies:
MeV MeV/Nucleon
Chemical reaction .000002 Varies
Beta decay 2 Varies
Alpha decay 6 .026
Superalpha decay 36 .16 5 or 6 alphas, in sequence. For example, Uranium-232
Superheavy decay 280 .98 Alpha, then fission. Superheavy elements with more than 108 protons
Neutron capture 8 Varies
Fission (Helium-3) .764 .19
Fission (Lithium-6) 4.783 .68
Fission (Beryllium-7) 1.64 .21
Fission (Boron-10) 2.34 .21
Fission (Uranium-235) 181 .77
Fission (Plutonium-239) 189 .79
Fission (Californium-252) 207 .82
Fusion of D+Li6 22.4 2.8
Beta decay, Beryllium-7 .547 .078
Beta decay, Sodium-22 2.842 .13
Beta decay, Scandium-46 2.366 .051
Beta decay, Cobalt-60 2.82 .047
 |
|---|
Half life Heat Decay Decay energy Melt Boil Obtainable by Formation rate
year Watt/kg MeV Kelvin Kelvin neutron transmutation
Tungsten-188 .191 148700 2β .349 3695 6203 * .173
Iridium-192 .202 * 193
Tantalum-182 .313 68866 β 1.814 3290 5731 * 6.42
Tungsten-181 .332 59200 EC 1.732 3695 6203 * .0239
Ruthenium-106 1.023 71200 2β 3.584 2607 4423 * .000198
Hafnium-172 1.87 11700 EC 1.835 2506 4876
Thorium-228 1.912 235000 5α+2β 34.784 2023 5061
Rhodium-101 4.07 9890 EC 1.980 2237 3968
Cobalt-60 5.27 19300 β 2.82 1768 3200 * 2.01
Osmium-194 6.02 4313 2β 2.33 3306 5285 * .130
Platinum-193 50 17.5 EC .057 2041 4098 * 3.81
Titanium-44 59.1 4318 EC & β+ 3.798 1941 3560
Uranium-232 68.9 7545 6α 40.79 1405 4404
Iridium-192m2 241 72 * 100
Thorium-229 7917 57.7 5α+3β 35.366 2023 5061
Protactinium-231 32600 16.2 6α+3β 41.33 1841 4300
Europium-152 13.5 8660
Europium-154 8.59 312
Europium-155 4.76 312
Meitnerium-285 2.24 1020000 α+fission 220 ~3300 ~5000
Darmstadtium-293 37.7 58900 β+fission 220 ~2600 ~4700
Darmstadtium-292 133 16700 α+fission 220 ~2600 ~4700
Darmstadtium-294 380 5820 α+fission 220 ~2600 ~4700
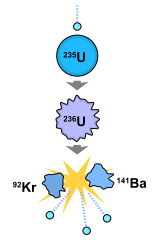 |
|---|
Half life Fission Fission Critical mass
year barn neutrons kg
Fermium-257 .275 2100 5.7
Einsteinium-254 .75 2900 4.2 9.9
Californium-251 900 4801 4.1 5.46
Californium-249 351 600 4.06 6.0
Curium-245 8500 2161 3.83 9.6
Americium-242m 141 7024 3.26 9.5
Plutonium-239 14100 748 2.89 10
Uranium-235 704000000 538 2.43 52
The transmutation options are:
Example Means
Add a neutron Uranium-238 → Uranium-239 Slow neutrons from fission. Thermal neutrons at 300 Kelvin and .025 eV
Subtract a neutron Thorium-232 → Thorium-231 Superfast neutrons from fusion. Neutron energy = 14.1 MeV
Beta decay Uranium-239 → Plutonium-239 Patience
Alpha decay Uranium-233 → Thorium-229 Patience
Fission Uranium-235 → Daughter nuclei + Neutrons
Accelerator substitution Lithium-6 + Deuteron → Beryllium-7 + Neutron
Accelerator fusion Berkellium-249 + Calcium-48 -> Tennessine-297
Some isotopes can be made with present technology and some need future technology. Also, the more energy you have, the more isotopes you can make.
Fission reactors are the easiest way to make isotopes. They produce slow neutrons that are captured by a target nucleus and they make isotopes that are "neutron heavy", or "neutronic".
Slow neutrons tend to stick to nuclei and superfast neutrons tend to eject neutrons from nuclei. Superfast neutrons can make protonic isotopes. Superfast neutrons are produced by fusion:
Deuterium + Tritium → Helium-4 (3.5 MeV) + Neutron (14.1 MeV)
Most isotopes can be made with slow or superfast neutrons. Many that can't can be made with accelerator substitution. A target nucleus is bombarded with high-energy particles such as deuterons, tritons, alphas, and He-3. For example,
Lithium-6 + Deuteron + 2 MeV → Beryllium-7 + Neutron
Isotopes can be made by fission, for example rhodium-103 and palladium-106. Rhodium and palladium are valuable catalysts.
Isotopes can be made by spallation, where a high-energy particle blasts nucleons off a nucleus.
Isotopes can be made by fusion, where 2 nuclei are collided at the resonance energy for fusion. This is how isotopes far beyond uranium are made.
Isotopes can be made by alpha decay. For example, slow neutrons turn thorium-232 into uranium-233, and then uranium-233 alpha decays to suburanics.
Slow neutrons tend to stick to nuclei and superfast neutrons tend to eject neutrons from nuclei. Superfast neutrons can make protonic isotopes. Superfast neutrons are produced by fusion:
Deuterium + Tritium → Helium-4 (3.5 MeV) + Neutron (14.1 MeV)
The cross sections for a fusion neutron hitting thorium-232 are (in barns):
Fusion neutrons Fission neutrons Thermal neutrons Energy threshold
14.1 MeV 2.4 MeV average .025 eV average MeV
Capture the neutron .0011 .096 7.299 0
Eject 1 neutron 1.786 .017 0 6.468
Eject 2 neutrons .522 .000072 0 11.61
Eject 3 neutrons 0 0 0 18.43
Fission .361 .080 .000054 0
Elastic collision 2.740 4.832 14.72 0
Inelastic collision .468 2.690 0 .0496
Total 5.859 7.717 22.02
For fusion neutrons, ejection is half the cross section.
Protonic isotopes can be produced by bombarding a target nucleus with a high-energy particle, such as a proton, deuteron, triton, He-3, or Alpha.
Beryllium-7 can't be made with fission or fusion neutrons, but it can be made by bombarding Lithium-6 with high-energy deuterons.
Target New High-energy Energy Cross section nucleus nucleus particle MeV barn Li6 Be7 d 2 .2 Mg24 Na22 d 7 .2 Th232 Ac227 t 75 .5 Th232 Ra228 t 100 .0018 Th232 Th228 d 83 .272 Th232 U-232 Alpha 38 .195 Th232 Ac225 p 150 .015 Th232 Ac226 p 150 .015 Th232 Ac227 p 150 .015 Th232 Th227 p 70 .040 Th232 Th228 p 60 .085
Suburanics can be made with a combination neutron addition and subtraction. Starting with thorium-232,
Th-232 → Th-231 → Th-230 → Th-229 → Th228
Th-232 → Th-233 → U-233 → U-232 → U-231 → U-230
Radium can be made by waiting for a thorium alpha decay.
Input Input half life Output Output half life
year
Thorium-230 75380 Radium-226 1599
Thorium-232 14000000000 Radium-228 5.75
Uranium-235 704000000 Protactinium-231 32600
Uranium-238 4470000000 Thorium-230 75380
Radium-226 can generate all the important suburanics with fission neutrons except for Uranium-230. The sequence is:
Radium-226 → Actinium-227 → Thorium-227 → Thorium-228 → Thorium-229 → Thorium-230 → Protactinium-231 → Uranium-232
Neutron capture is slow. Some isotopes need a high neutron flux. A prime goal is maximizing neutron density, and a future civilizations will have neutron densities than today.
The neutron capture rate is:
Neutron density = D = e16 neutrons/meter3 Typical for a fission reactor Neutron speed = V = 2190 meter/second Thermal neutron at 300 Kelvin Neutron flux = F = D V =2.2e19 neutrons/meter2/second Typical for a fission reactor Neutron capture cross section = A = e-28 meter2 = 1 barn Typical cross section Transmutation time = T = (DVA)-1 = 4.6e8 seconds = 14 years
Many isotopes need a small transmutation time. This can be done by increasing the neutron flux or by increasing the capture cross section. The slower the neutron, the larger the cross section.
For neutrons below 100 eV, it's often the case that "VA" is constant as a function of V.
At Oak Ridge National Laboratory, the High Flux Isotope Reactor has a flux of 2.5e19 neutrons/meter2/second.
The Oak Ridge Spallation Source produces neutron pulses with e21 neutrons/meter2/second.
Neutrons are made by hitting beryllium-9 with high-energy alphas. The alpha source is often polonium-210.
Beryllium-9 + &alpha → Carbon-12 + Neutron
Continuous flux Pulse flux Continuous power
Neutron/meter2/s Neutron/meter2/s MWatt
Supernova - e36 e30 neutrons/meter3
Fission bomb e28
Oak Ridge fission reactor 2.5e19 - 75
Oak Ridge spallation source 3e18 2e21 1.4
ITER fusion reactor 2e18 - 500
Stellar S process e15
Californium-252 e13
Polonium-210 + Beryllium-9 e12 - Polonium-210 half life = .379 years
Fusor e9 - Use high voltage to fuse D+T
Radium-226 + Beryllium-9 e8 - Radium-226 half life = 1599 years
Earth surface .0065
Deep underground e-9
In many cases, the slower the neutron, the higher the capture cross section.
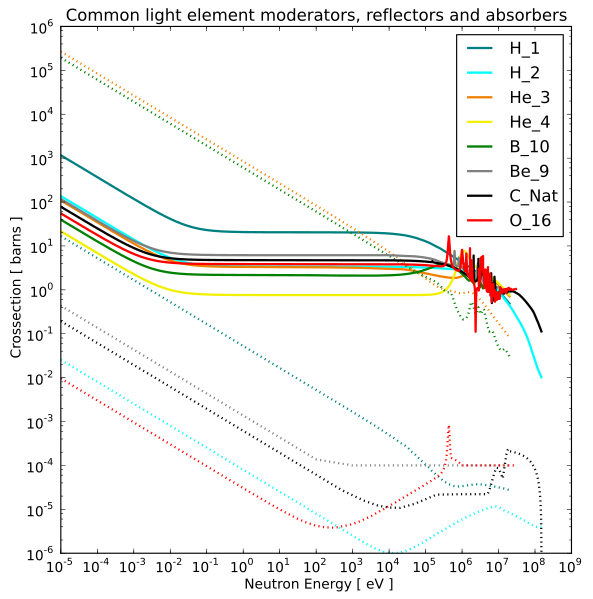 |
|---|
Solid lines are scattering cross sections and dotted lines are capture cross sections.
Kelvin
Water melt 273
CO2 sublime 195
Argon boil 87
Nitrogen boil 77
Neon boil 27
Hydrogen boil 21
Helium-4 boil 4.2
Helium-3 boil 3.2
Helium-3 evaporative cooling .3
Dilution refrigerator .002 Uses helium-3 and helium-4
Nuclear demagnetization, typical .000001
Laser cooling, typical .000001
Nuclear demagnetization, record low .0000000001
Laser cooling, record low .000000000038
Cold neutrons bounce off walls. The critical temperature for various materials is:
Neutron Neutron Neutron
temperature temperature speed
neV Kelvin meter/second
Nickel-58 335 .0039 8.14
Diamond 304 .0036 7.65
BeO 261 .0031 6.99
Nickel 252 .0030 6.84
Beryllium 252 .0030 6.84
Graphite 180 .0021 5.47
This is within reach of a dilution refrigerator, which can reach .002 Kelvin. It's also within reach of magnetic trapping.
An advanced civilization will have unlimited helium-3 and can make a large dilution refrigerator. It can make a neutron wall trap that can collect all the outgoing neutrons from a fission reactor and funnel them to a focus. The only limit on neutron density is neutron degeneracy, which is 1022 neutrons/meter3 at .003 Kelvin.
Ultracold neutrons can be trapped magnetically. Neutrons have a magnetic moment of 50 neV/Tesla. For a 3 Tesla field, this is the same temperature range as a dilution refrigerator and as for wall trapping.
Magnetic fied (Tesla)
Neodymium magnet 1.25
Magnetic resonance imaging 7
Superconducting magnet max, continuous operation 32
Resistive magnet max, continuous operation 38
Pulsed magnet max, non-destructive 100
Pulsed magnet max, destructive 2000
White dwarf 1000
Neutron star 10000000
Magnetar 10000000000
Magnetar max 100000000000
Neutron density is limited by degenercy pressure. For neutrons,
Type Energy Temperature Wavelength Speed Degenerate density
eV Kelvin Angstroms Meter/second #/meter3
Fusion 14100000 .000061 51900000
Fast 2000000 .00016 19600000
Thermal .0253 294 1.45 2200 3.3⋅1029
Cold .00036 4.2 12.1 263 5.6⋅1026
Ultracold .00000022 .0025 497 6.4 8.1⋅1021
Fast neutrons are from fission.
Fusion neutrons are from the fusion of D+T->He4+n, which produces a 14.1 MeV neutron.
 |
|---|
Half life Neutron capture (barns)
Osmium-192 Stable 3.12 Input isotope
Osmium-193 1.25 days 38.0 Hurdle isotope
Osmium-194 6.0 years .043 Output isotope
If there is a hurdle isotope, the formation rate of the output isotope is proportional to:
Hurdle half life = T Hurdle neutron capture cross section = σc Hurdle fission cross section = σf Natural fraction = F Fraction of the natural element that can transform to the output isotope Capture fraction = C = σc/(σc+σf) Formation rate of output isotope = Q = T σc F C
This is for the case of an output isotope with a long half life and a hurdle isotope with a short half life.
If the output isotope has a short half life, then the output isotope is the hurdle. "T" becomes the half life of the output isotope and σc becomes the capture cross section for the isotope preceeding the output isotope.
If the hurdle time is more than 1 year, we use a time of 1 year.
 |
|---|
In the plot, "Island #1 and #2" are the nuclear islands of stability. Island #1 needs a formation time of order .1 seconds, and island #2 needs .01 seconds, and superheavy isotopes (udQM regime) need .001 seconds.
The isotopes with hurdles to their formation are:
Output Output Hurdle Capture Natural Capture Production Hurdle
Half life half life rate
year year barn fraction fraction barn*year
Europium-152 13.5 13.5 9100 .952 1 8660 Europium-151
Europium-154 8.59 8.59 312 1 1 312 Europium-153
Europium-155 4.76 4.76 312 1 1 312 Europium-153
Iridium-192 .202 .202 954 1 1 193 Iridium-192
Iridium-192m2 241 241 100 1 1 100 Iridium-192m2
Tritium 12.3 Stable 940 .0759 1 71 Lithium-6
Thulium-170 .352 .352 105.6 1 1 37.2 Thulium-170
Thulium-171 1.91 .352 87.6 1 1 30.8 Thulium-170
Caesium-134 2.06 2.06 30.3 1 1 30.3 Caesium-137
Americium-242m 141 141 84 1 .10 8.4 Americium-242m
Tantalum-182 .313 .313 20.5 1 1 6.42 Tantalum-182
Platinum-193 50 Stable 10.0 .381 1 3.81 Platinum-192
Cobalt-60 5.27 5.27 2.007 1 1 2.007 Cobalt-60
Carbon-14 5730 Stable 1.931 .996 1 1.92 Nitrogen-14
Plutonium-238 87.7 2144000 171 .0072 .146 1.23 Neptunium-237
Argon-39 269 Stable .8 1 1 .8 Argon-38
Cadmium-109 1.26 Stable 1.1 .518 1 .57 Cadmium-108
Scandium-46 .229 .229 2.366 1 1 .542 Scandium-46
Iron-55 2.737 2.737 2.25 .0585 1 .36 Iron-55
Deuterium Stable Stable .333 .99985 1 .333 Proton
Zinc-65 .667 .667 .93 .492 1 .305 Zinc-65
Tungsten-188 .191 .00270 64.1 1 1 .173 Tungsten-187
Californium-252 2.64 2.64 2.49 1 .0673 .168 Curium-248
Osmium-194 6.0 .00343 38.04 1 1 .130 Osmium-193
Technetium-99 211000 211000 .132 .903 1 .119 Technetium-99
Strontium-90 28.9 .138 .420 1 1 .0580 Strontium-89
Tungsten-181 .332 .332 60 .0012 1 .0239 Tungsten-181
Promethium-145 17.7 .931 .7 .0308 1 .022 Samarium-145
Samarium-145 .931 .931 .7 .0308 1 .022 Samarium-145
Californium-254 .166 .049 20.0 1 .0151 .0148 Californium-253 1303
Polonium-210 .379 .379 .0338 1 1 .0128 Polonium-210
Iodine-131 .0220 Stable .29 .341 1 .00218 Tellurium-130
Ruthenium-106 1.023 .000507 .390 1 1 .000198 Ruthenium-105
Argon-42 32.9 .000208 .509 1 1 .000106 Argon-41
Silicon-32 153 .000299 .107 1 1 .000032 Silicon-31
Curium-250 8300 .000122 1.60 1 .138 .0000027 Curium-249 10
Lead-210 22.3 .000371 .00143 1 1 .00000053 Lead-209
Caesium-137 30.2 .0360 Unknown 1 1 Caesium-136
Output Output Hurdle Capture Natural Capture Production Hurdle
Half life half life rate
year year barn fraction fraction barn*year
Einsteinium-257 .0211 .000048 Unknown Unknown Einsteinium-256
Einsteinium-259 .0180 .000074 Unknown Unknown Einsteinium-258
Fermium-257 .275 .000300 Unknown Unknown Fermium-256
Subjecting natural europium to neutrons for a short time produces mostly europium-152.
Subjecting natural europium to neutrons for a medium time produces mostly europium-154.
Subjecting natural europium to neutrons for a long time produces 2/3 europium-154 and 1/3 europium-155.
Half life Neutron capture Natural Energy Gamma max Decay
year barn fraction MeV MeV
Europium-151 Stable 9100 .478 - -
Europium-152 13.5 11800 0 1.86 1.408
Europium-153 Stable 312 .522 - -
Europium-154 8.59 1663 0 1.968 1.274
Europium-155 4.76 3843 0 .252 .147
Europium-156 .0416 100 0
Europium-157 .00174 0
Thulium-169 Stable 105.6 1 -
Thulium-170 .352 87.6 0 .968 β
Thulium-171 1.91 9.90 0 .096 β
Promethium-143 .725 3.07 0 β+
Promethium-144 .994 15.09 0 β+
Promethium-145 17.7 5.28 0 .164 .072 EC
Promethium-146 5.53 8.41 0 1.495 1.189 EC
Promethium-147 2.623 167.2 0 .224 .224 Β 72 barn to Pm-148m. 82 barns to Pm-148
Promethium-148 .0147 0 β
Promethium-148m .113 0 β
Neodymium-142 Stable 18.7 .272
Neodymium-143 Stable 337 .112
Neodymium-144 Stable 3.6 .238
Neodymium-145 Stable 42 .0829
Neodymium-146 Stable 1.4 .172
Neodymium-147 .0301 142.9 0 β
Neodymium-148 Stable .0575
Neodymium-149 .000194 0 β
Neodymium-150 Stable .0563
Samarium-144 Stable .7 .0308
Samarium-145 .931 280.3 0 .617 .061 EC
Samarium-146 68000000 .382 0
Samarium-147 Stable 57 .150
Samarium-148 Stable 24 .112
Samarium-149 Stable 42080 .138
Samarium-150 Stable 104 .0737
Samarium-151 90 14070 0
Samarium-152 Stable 206 .267
Samarium-153 .00528 0
Samarium-154 Stable 8.4 .227
Making transuranics takes many neutrons. Also, fission can halt the process. When going from uranium-238 to californium-251, the fraction of isotopes that make it to californium-251 is .0000151.
There are no hurdles with short half lives until fermium-258, with a half life of .000370 seconds.
The sequence for making transuranics is:
Half life Fission Capture Capture Cumulative Decay
year barn barn fraction fraction
Uranium-238 4470000000 .000010 2.68 1 1 α
Plutonium-239 14100 748 1017 .58 .58 α
Plutonium-240 6561 .030 290 1 .58 α
Plutonium-241 14.3 937 363 .28 .16 β
Plutonium-242 373000 .0026 18.5 1 .16 α
Americium-243 7370 .2 75.3 1 .16 α
Curium-244 18.1 1.1 13 .92 .16 α
Curium-245 8500 2161 383 .151 .147 α
Curium-246 4730 .17 1.36 .89 .0222 α
Curium-247 15700000 82 58 .41 .0198 α
Curium-248 340000 .34 2.49 .88 .00811 α
Californium-249 351 1666 483 .22 .00714 α
Californium-250 13.08 112 1701 .94 .00157 α
Californium-251 900 4894 2849 .37 .00148 α
Californium-252 2.64 33.0 20.4 .38 .000546 α
Californium-253 .049 1303 19.95 .0151 β
Californium-254 .166 2.001 4.51 .69 Fission
Einsteinium-255 .109 .503 55.4 1 β
Fermium-256 .000300 Unknown Unknown Unknown α
Fermium-257 .275 2951 3.003 .00102 α
Fermium-258 .000370 Unknown Unknown Unknown Fission
Fermium-259 1.5 Unknown Unknown Unknown Fission
Fermium-260 .004 Unknown Unknown Unknown Fission
A neutron could either be captured or it could trigger fission. "Capture fraction" is the fraction of capture events.
"Cumulative fraction' is the product of capture fractions up to the given isotope.
Energy per neutron = E = 200 MeV/neutron Neutron mass = M = 1.67e-27 kg Neutron energy/mass = e = E/M = 1.9e16 Joule/kg Electricity energy/$ = L = 30 MJoule/$ Neutron price/kg = P = e/L = 640 M$/kg
A neutron can turn uranium-238 into plutonium-239. The energy cost of plutonium-239 is 2.7 M$/kg.
Actinides beyond plutonium take many neutrons to make. It takes 14 neutrons to get from uranium-238 to californium-252.
A calutron separate isotopes with a magnetic field.
Protons Price/kg World produce America stockpile Natural fraction
M$/kg kg/year kg
1 Hydrogen-2 .004 1000000 .00015
1 Hydrogen-3 30 10 30 0
2 Helium-3 1 10 30 .000002
2 Helium .000024 50000000
3 Lithium .000070 36000000
3 Lithium-6 .06 .0759
4 Beryllium .00150 425000 All beryllium-9
4 Beryllium-7 0
5 Boron-10 .20
6 Carbon-12 .12 .989
6 Carbon-14 .000000000001
21 Scandium-46
27 Cobalt-60
38 Strontium-90 .01
55 Caesium-137
63 Europium-152
63 Europium-154
77 Iridium-192
77 Iridium-192m
84 Polonium-209 50000000
84 Polonium-210
88 Radium-226 .1 0 30
88 Radium-228 .1 0 30
89 Actinium-227 1
90 Thorium .000290 10000000
90 Thorium-228
90 Thorium-229
90 Thorium-230
90 Thorium-231
91 Protactinium-231 .28
92 Uranium .000101 70000000
92 Uranium-230
92 Uranium-232
92 Uranium-233 2000
92 Uranium-235 .1 480000 .0072
93 Neptunium-237 .66
94 Plutonium-238
94 Plutonium-239 6.5 80000
95 Americium-141 .7 10
95 Americium-142m
95 Americium-143 .7 10
95 Curium-242 1 10
95 Curium-243
95 Curium-244 185 .17 10
95 Curium-245
95 Curium-246
95 Curium-247 .1
95 Curium-248 160000 .0001
96 Curium-250
97 Berkelium-249 185000 .020 N/A
98 Californium-249 185000 .0005
98 Californium-250
98 Californium-251
98 Californium-252 60000 .5 N/A
99 Einsteinium-253 .01 N/A
99 Einsteinium-254 .000001 N/A
100 Fermium-257
Power Power Distance
Watts Sun=1 light year
Human civilization, 1800 6.0e11
Human civilization, 1900 1.3e12
Human civilization, 2023 2.0e13
Solar power hitting Earth 1.7e17
Sun 3.8e26 1 0
Star Sirius 9.7e27 25.4 8.6
Star Aldebaran 2.0e29 520 65
Star Spica 7.8e30 20500 280
Star Naos 3.1e32 813000 1080
Star eta Carine 1.7e33 4600000 7500
Milky Way central region 1 e35 26000 Stars within 10 light years of the center
Galacy Centaurus A AGN 11000000 Nearest active galactic nucleus
Milky Way total 5 e36 -
Virgo central galaxy 5 e37 53500000
Quasar Markarian 231 1.4e39 581000000 Nearest quasar
Quasar Ton 618 4 e40 18200000000 Brightest quasar
Galaxy W2246-0526 1.3e41 12600000000 Brightest galaxy
Isotopes that decay by alpha and don't produce betas or gammas have value because they can be around humans. The pure alpha isotopes are:
Half life Power/Mass Obtainable by
year Watt/kg neutron transmutation
Fermium-257 .275 * Fm257 -> Es253 -> Bk249
Polonium-210 .379 144000 *
Curium-242 .446 124000
Polonium-208 2.898
Curium-244 18.1 2823 * Cm242 -> Pu240 -> U236
Curium-243 29.1 1885 * Cm243 -> Pu239 -> U235
Gadolinium-148 75 800
Plutonium-238 87.7 578 *
Polonium-209 125.2 Po209 -> Pb205 -> Tl205 The electron capture has a half life of 15 Myr
Americium-241 432 114 *
A nuclear reactor needs materials with a low neutron capture cross section. Zirconium is the favored structural metal.
Neutron capture Natural Neutron Density Atom Stopping
fraction scatter density power
barn barn g/cm3
Oxygen .00028 -
Carbon .0035 -
Helium .007 -
Beryllium .0092 -
Bismuth .034 -
Neon .04 -
Magnesium .063 -
Lead .171 -
Zirconium .184 -
Aluminum .232 -
Hydrogen .332 -
Niobium 1.15 -
Ruthenium 2.56 -
Molybdenum 2.6 -
Nickel 4.49 -
Platinum 10 -
Osmium 15 -
Tungsten 18.3 -
Tantalum 20.6 -
Hafnium 104 -
Hydrogen-3 0 0 2.178
Helium-4 0 1.000 .961 .145 .036 .036
Carbon-14 .000000310 0 3.152 4.11 .294 .93
Nitrogen-15 .000024 .004 5.293
Beryllium-10 .000100 0 6.291 2.06 .206 1.30
Oxygen-18 .000141 .00205 3.987
Oxygen-16 .000190 .998 4.498
Lead-208 .000230 .524 12.94 11.34 .055 .72
Hydrogen-2 .000550 .000145 4.705
Carbon-13 .00137 .011
Carbon-12 .00389 .989
Boron-11 .00508 .80
Beryllium-9 .0076 1 1.85
Zirconium-90 .0107 .514
Magnesium-26 .0382 .110
Lithium-7 .0454
Molybdenum-92 .0614 .146
Ruthenium-106 .146 0
Titanium-50 .179 .0518
Aluminum-27 .231 1
Oxygen-17 .244
Hydrogen-1 .333 .9999 32.81
Chromium-54 .36 .0236
Platinum-196 .72 .253
Iron-58 1.28 .0028
Nickel-64 1.52 .00926
Osmium-192 3.12 .41
Tungsten-183 10.1 .143
Hafnium-180 12.92 .351
Tantalum-181 20.5 .999
The best isotope for neutron shielding is gadolinium-157, with a neutron capture cross section of 259000 barns. Natural gadolinium is a mix of isotopes with an overall cross section of 49000 barns.
Elements and isotopes with a high neutron capture cross section are:
Neutron capture Price/kg Quality
barns $/kg barns/$
Gadolinium 49000 29 2450
Samarium 5922 13.6 740
Europium 4600 287 15
Cadmium 2450 2.7 1220
Boron 767 3.7 183
Lithium 70.5 70 .35
Xenon-135 2650000 Half life = .00104 year
Zirconium-88 861000 Half life = .228 year
Gadolinium-157 259000
Gadolinium-155 61100
Beryllium-7 56800
Samarium-149 42080
Cadmium-113 30000
Europium-151 9100
Helium-3 5333
Boron-10 3835
Lithium-6 940
Neutrons can be generated by bombarding beryllium-9 with alpha particles.
α + Beryllium-9 → Carbon-12 + Neutron
 |
|---|
Half life Decay Gamma energy Obtainable by neutron transmutation
year MeV
Antimony-124 .165 Beta 2.09 Most gammas are 1.69 MeV. Max 2.09 MeV
Cobalt-60 5.26 Beta 1.33 *
Sodium-22 2.6 Beta+ 1.28
Hafnium-178m 31 Gamma .507 *
 |
|---|
Lithium-6 + Neutron → Helium-4 + Tritium Cross section of 940 barns
Half life Obtainable by
year neutron transmutation
Fluorine-18 .000209
Molybdenum-99 .00752 * Decays to Technetium-99m, which has a half life of 6.01 hours
Thallium-201 .00832
Iodine-131 .022 *
Iodine-125 .0362
Palladium-103 .0465 *
Strontium-82 .0694 Decays to Rubidium-82
Iridium-192 .202 *
Strontium-89 *
Samarium-153 *
Rhenium-186 *
Lutetium-177 *
Bismuth-213 *
Lead-212 *
Radium-223
Boron-10 Stable Natural
Gadolinium-157 Stable Natural
 |
|---|
Some isotopes undergo 5 or 6 alpha decays, such as uranium-232. This is the red line in the plot.
U-232 → Th-228 → Ra-224 → Rn-220 → Po-216 → Pb-212 → Bi-212 → Po-212 → Pb-208
The double-decay nuclei with half lives between 1 month and 100 years are:
1st decay 2nd decay Total 1st 2nd Obtainable by neutron transmutation
half life half life energy energy energy
year year MeV MeV MeV
Osmium-194 → Ir-194 → Pt-194 6.02 .00022 2.330 .096 2.234 *
Tungsten-188 → Re-188 → Os-188 .191 .00194 2.469 .349 2.120 *
Rhenium-184m → Re-184 → Os-184 .463 .104 .646 .188 .458 *
Hafnium-172 → Lu-172 → Yb-172 1.87 .0283 1.835 .338 1.497
Fermium-247 → Es-253 → Cm-249 .275 .0560 *
Rhodium-102m → Rh-102 → Ru-102 3.742 .567 1.509 .141 1.268
Einstein-254 → Cf-250 → Cm-246 .755 13.3 *
Titanium-44 → Sc-44 → Ca-44 59.1 .00046 3.798 .146 3.652
Strontium-90 → Y-90 → Zr-90 28.9 .00731 2.826 .546 2.280 *
Iridium-192m → Ir-192 → Pt-192 241 .202 1.615 .155 1.460 *
Tin-121m → Sn-121 → Sb-121 43.9 .00308 .396 .0063 .390 *
Argon-42 → K-42 → Ca-42 32.9 .00141 4.124 .599 3.525 *
Silicon-32 → P-32 → S-32 153 .0391 1.919 .21 1.709 *
Germanium-68 → Ga-68 → Zn-68 .742 .000129
 |
|---|
Melt Boil Density
Zirconium 2128 4650
Niobium 2750 5017
Molybdenum 2896 4912
Technetium 2430 4538
Ruthenium 2607 4423
Rhodium 2237 3968
Lutetium 1925 3675
Hafnium 2506 4876 13.3
Tantalum 3290 5731 16.7
Tungsten 3695 6203 19.2
Rhenium 3459 5309 21.0
Osmium 3306 5285 22.6
Iridium 2719 4403 22.6
Platinum 2041 4098 21.4
Thorium 2023 5061
Protactinium 1810 4300
Uranium 1405 4404 19.1
Neptunium 912 4447
Curium-240 produces 2 alphas with short half lives and becomes Uranium-232, which has a long half life. Uranium-232 produces an alpha with a long half life and then 5 more alphas with short half lives. It can be used as an alpha rocket, where the first two decays give it a fast launch, and the remaining decays power it for a long time. The decay sequence of Curium-240 is:
Half life
year
Curium-240 .0739 Alpha
Plutonium-236 2.858 Alpha
Uranium-232 68.9 Alpha
Thorium-228 1.912 Alpha
Radium-224 .00986 Alpha
Radon-220 .0000018 Alpha
Polonium-216 .000000005 Alpha
Lead-212 .00121 Alpha
Bismuth-212 .00012 Beta
Tellurium-208 .0000057 Alpha
Lead-208 Stable Beta
Cross sections for fusion neutrons at 14.1 MeV, in millibarns. All columns are fusion neutrons except the last column, which is fast fission neutrons. For fusion neutrons, the usual outcome is for neutrons to be subtracted from the target nucleus.
Thorium is more likely to lose neutrons than uranium.
(n,2n) (n,3n) (n,fission) (n,inelastic) (n,G) (n,2n)
fission
Actinium-227 2170 353 62.8 445 1.119 15.87
Thorium-228 1897 85.8 598 498 1.23 7.39
Thorium-229 1717 92.7 864 346 1.19 33.5
Thorium-230 1856 244 564 424 .96 12.2
Thorium-231 1685 336 658 349 1.21 42.7
Thorium-232 1786 522 361 468 1.14 16.8
Uranium-320 174 .0126 2518 416 .78 .138
Uranium-321 75 .000028 2474 403 .69 2.08
Uranium-322 401 .77 2570 420 .82 .760
Uranium-233 181 .271 1.84 368 .58 1.84
Curium-242 231 .120 2907 359 1.19 .921
The prompt kinetic energy released by fission is:
Fission energy (MeV)
Actinium 168
Thorium 172
Protactinium 177
Uranium 181
Neptunium 185
Plutonium 189
Americium 195
Curium 198
Berkelium 203
Californium 207
 |
|---|
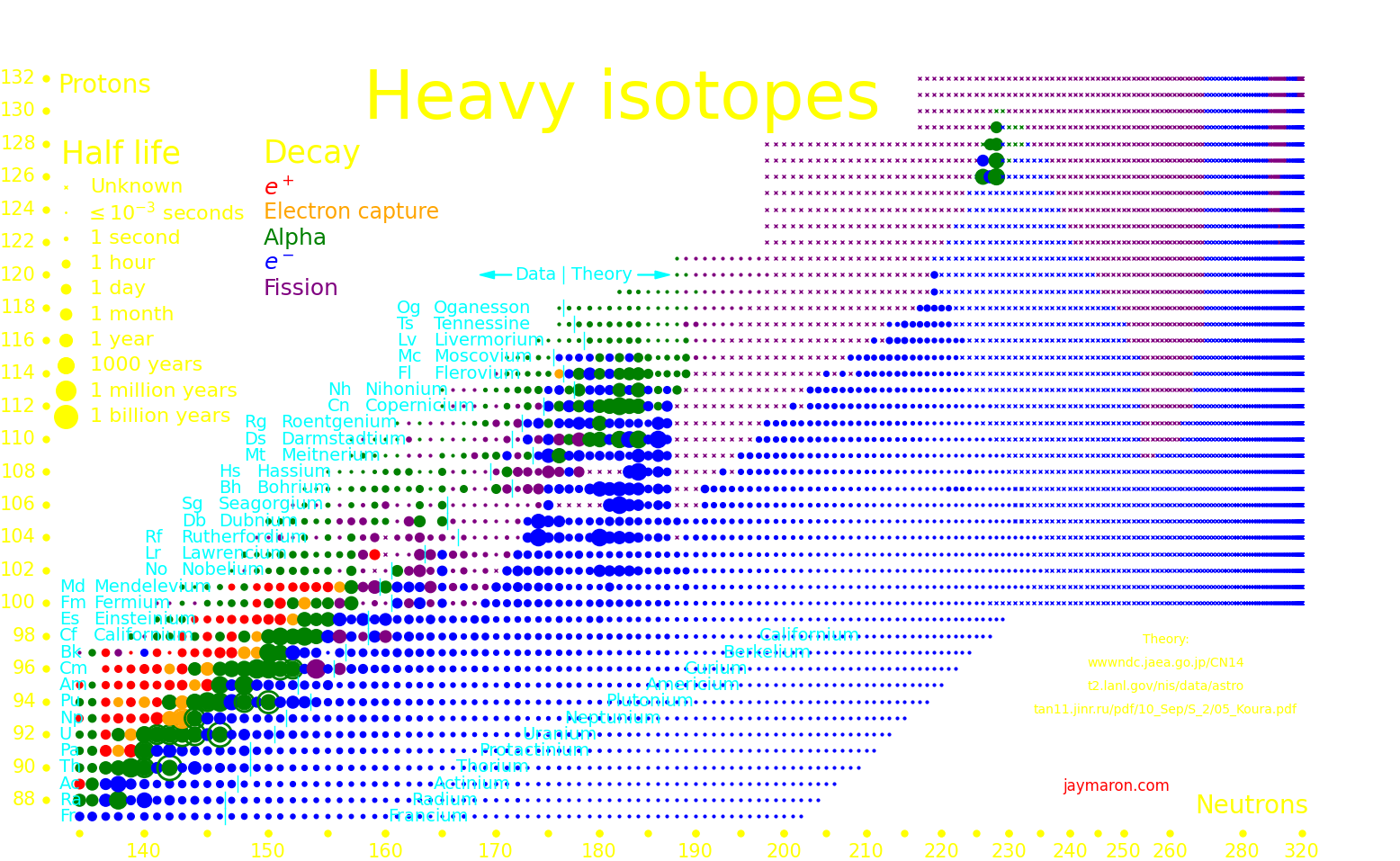
There is a hypothetical "island of stability" around atomic number 112 where nuclei may be long-term stable. A second island may exist at atomic number 126.
Experiments can only measure the longest-lived isotope up to a proton number of 105, and beyond that we plot theory. Theoretical half lives are uncertain by an order of magnitude.
 |
|---|
 |
|---|
It's possible that for large nucleon number, larger than around 300, that the nucleus transitions to a lower-energy state, called "Up down quark matter", or "udQM". The existence of udQM is unresolved. Theory is uncertain, and it hasn't been experimentally produced. The largest nucleus that's been produced is oganesson-294, with 118 protons and 294 nucleons. It shows no sign of udQM, so if udQM exists, it's beyond oganesson.
If udQM nuclei exist, they could potentially be long-term stable. They don't fission because it would take the nucleus to a higher-energy state. They decay by alpha until they're too light to be udQM, at which point they fission.
If udQM nuclei exist, then there may exist long-lived elements from Z=140 to way beyond. These are "continental elements".
The largest nucleus that standard nuclei can make has Z=140. Nuclei larger fission instantly. The only way that nuclei with Z>140 can exist is if udQM exists.
In the isotope table, we use experimental data from Wikipedia if it exists, otherwise we use theory from https://wwwndc.jaea.go.jp/CN14 and https://t2.lanl.gov/nis/data/astro. Nobel gases have a closed shell of electrons, and the shell numbers are called "magic numbers". The magic numbers for electron shells are:
Helium 2 Neon 10 Argon 18 Krypton 36 Xenon 54 Radon 86 Oganesson 118
The magic numbers for neutrons are 2, 8, 20, 28, 50, 82, 126, 184. The magic numbers for protons are the same as for neutrons, up to 82, and the next proton magic numbers are 114, 126, 154, and 164. Nuclei that are magic for both protons and neutrons are:
Protons Neutrons
Helium-4 2 2
Oxygen-16 8 8
Calcium-40 20 20
Calcium-48 20 28
Lead-208 82 126
Flevorium-298 114 184 Undiscovered
Unnamed 126 216 Undiscovered
Unnamed 126 228 Undiscovered
Unnamed 154 308 Undiscovered
Unnamed 164 308 Undiscovered
Unnamed 164 318 Undiscovered
Unnamed 164 406 Undiscovered
The nuclei from helium-4 to lead-208 are stable.
 |
|---|
A Fermi gas with a Fermi energy of 335 neV has a density of 1.39e23 particles/meter3
Fermi number density = n = 16π/3 λ-3= 1.39e23 particles/meter3
Fermi wavelength = λ = 4.94e-8 meter
Fermi momentum = Q = 1.34e-26 kg*meter/second
Planck constant = h = Q ℏ = 6.626e-34 Joule*second
Fermi energy = E = Q2/(2m) = ℏ2/(2m) (3π2n)2/3
= 335 neV
Neutron mass = m = 1.675e-27 kg
 |
|---|
Full list.
Half life Heat Decay Electron Elect Gamma Form rate Obtainable Decay
max ave max by neutron
year Watt/kg MeV MeV MeV MeV barn*year transmute
Neutron .000027 .782 .782 * β Half life = 879 seconds
Einsteinium-253 .0560 * α
Tungsten-188 .191 19920 .349 8.49 * 2β
Californium-254 .166 11200000 207 .0102 * Fission
Iridium-192 .202 77147 1.460 134 * β
Scandium-46 .229 460000 2.366 .357 .112 1.121 .38 * β
Sulfur-35 .239 .167 .0488 * β
Fermium-247 .275 * α
Tantalum-182 .313 65260 1.814 4.45 * β
Tungsten-181 .332 59100 1.732 .0166 * EC
Thulium-170 .352 11800 .968 .968 .968 37.2 * β M 73% e- .968 MeV. 22% e- .884 MeV & .084 MeV gamma
Polonium-210 .379 139000 5.41 0 .0089 * α M
Calcium-45 .445 .257 .257 .257 * β
Curium-242 .446 * α
Gadolinium-153 .658 .484 .173 * EC
Zinc-65 .668 63900 1.352 .21 * β+
Einsteinium-254 .755 * α
Samarium-145 .931 * EC
Ruthenium-106 1.023 67700 3.584 .00014 * 2β
Cadmium-109 1.267 * EC
Thulium-171 1.91 .0965 30.8 * β
Caesium-134 2.06 15300 2.059 21 * β
Promethium-147 2.6 1200 .224 .86 * β
Californium-252 2.64 41400 12.33 0 * α 96.9% (6.12 Mev). Fission 3.09% (207 MeV)
Iron-55 2.74 3140 .231 .091 * EC
Thallium-204 3.78 .766 * β
Europium-155 4.76 705 .252 * β
Cobalt-60 5.27 18300 2.82 1.39 * β
Promethium-146 5.5 1.495 * EC 66%, e- 34%
Osmium-194 6.02 4313 2.330 .090 * 2β
Europium-154 8.59 3049 1.968 216 * β
Barium-133 10.51 758 .517 .0049 * EC
Tritium 12.33 1031 .0186 .0186 .0057 49 * β
Europium-152 13.5 1858 1.86 6005 * β & EC
Californium-250 13.08 * α
Cadmium-113m 14.1 340 .264 * β
Plutonium-241 14.3 3050 4.90 * β
Promethium-145 17.7 131 .164 * EC
Curium-244 18.1 * α
Lead-210 22.3 2907 9.100 0 .00000037 * α
Strontium-90 28.9 460 2.826 .29 * β, β M
Curium-243 29.1
Caesium-137 30.1 583 1.176 * β
Hafnium-178m2 31 930 2.446 * γ
Argon-42 32.9 .599 * β
Tin-121m 43.9 153 .396 .088 * IT, β
Platinum-193 50 17.5 .057 6.93 * EC
Plutonium-238 87.7 578 5.59 0 .125 * α
Samarium-151 90.0 11.6 .077 34 * β
Nickel-63 100.1 5.52 .066 .066 .017 .066 9.96 * β
Americium-242m 141 725 12.33 0 5.8 * 2α
Silicon-32 153 804 1.92 2.22 * β, β
Iridium-192m2 241 72 1.628 69 * β
Argon-39 269 .566 * β
Californium-249 351 * α
Americium-241 432.2 114 * α M
Californium-251 900 * α
Curium-246 4760 * α
Carbon-14 5730 3.99 .156 .156 .049 * β
Plutonium-240 6561 5.256 * α
Americium-243 7370 * α+β
Curium-250 8300 170 148 .000019 * Fission 74%, Alpha 18%, Beta 8%
Curium-245 8500 * 2α+β
Plutonium-239 24110 * α
Technetium-99 211000 .003 .294 * β
Curium-248 348000 * α
Plutonium-242 375000 * α
Beryllium-10 1390000 .556 * β
Technetium-97 4200000 * EC
Technetium-98 4200000 * β
Curium-247 15600000 * 3α+2β
Uranium-236 23420000 * α
Plutonium-244 81300000 * 3α+2β
Thorium-227 .0512 9194000 36.14 5α+2β
Uranium-230 .0554 9280000 6α+2β
Thorium-228 1.912 235000 34.784 5α+2β
Radium-228 5.75 90660 40.198 5α+4β
Actinium-227 21.8 21600 36.18 5α+3β
Uranium-232 68.9 7545 40.79 6α+2β
Radium-226 1599 286 34.958 5α+4β
Thorium-229 7917 57.7 35.366 5α+3β
Protactinium-231 32600 16.2 41.33 6α+3β
Thorium-230 75380 6.78 39.728 6α+4β
Uranium-233 159200 * 6α+2β
Uranium-234 245500 7α+4β
Neptunium-237 2144000 8α+4β
Curium-247 15600000 * 3α+2β
Uranium-235 703800000 * 7α+4β
Uranium-238 4468000000 .00010 51.771 * 8α+6β
Thorium-232 14050000000 47.655 * 6α+2β
Mendelevium-260 .076 Fission
Mendelevium-258 .141 α
Beryllium-7 .146 1822000 .547 EC
Cobalt-56 .212 β+
Rhenium-184m .463 16000 IT, β
Thulium-168 .255 β+
Gold-195 .510 .227 .210 EC
Cobalt-57 .744 .836 .137 EC
Manganese-54 .855 64400 EC
Vanadium-49 .901 .602 EC
Californium-248 .913 α
Einsteinium-252 1.29 0 α, EC
Lutetium-173 1.37 .630 EC
Tantalum-179 1.82 1.060 EC
Plutonium-236 2.858 α
Hafnium-172 1.87 11700 1.835 EC
Sodium-22 2.6 68700 2.842 β+ or EC
Polonium-208 2.99 0 α
Rhodium-101 3.3 EC
Lutetium-174 3.31 β+
Rhodium-102m 3.742 β+
Rhodium-101 4.07 9890 1.980 EC
Niobium-93m 16.1 IT
Bismuth-207 31.55 2.397 β+
Europium-150 36.9 2.259 β+
Titanium-44 59.1 4318 3.798 EC, β+
Terbium-157 71.0 11.0 .060 β+
Gadolinium-148 75 800 0 α
Polonium-209 125.2 0 α
Terbium-158 180 β+
Iridium-192m2 241 IT, β
Holmium-163 4570 EC
Darmstadtium-293 37.7 58900 220 β+fission Theoretical
Darmstadtium-292 133 16700 220 α+fission Theoretical
Copernicium-294 355 6230 220 α+fission Theoretical
Darmstadtium-294 380 5820 220 α+fission Theoretical
Argon-37 .0824 .814 0 * EC
Argon-39 269 * β
Argon-42 32.9 * β
Krypton-85 10.78 * β
Xenon-127 .0994 .662 0 .618 * EC
Half life Heat Decay Electron Elect Gamma Form rate Obtainable Decay
max ave max by neutron
year Watt/kg MeV MeV MeV MeV barn*year transmute
A neutron has a half life of 610.1 seconds and a decay energy of .782 MeV.
Full list.
Half life Power/mass Decay Gamma Formation Obtainable by Decay
energy max rate neutron
year Watt/kg MeV MeV barn*year transmutation
Nickel-63 100.1 5.52 .017 .017 2.5 * β
Tritium 12.33 315 .0186 .0186 71 * β
Rubidium-83 .236 .910 .0322 0 EC
Arsenic-73 .220 .341 .0534 0 EC
Terbium-157 71.0 11.0 .060 .054 0 EC
Samarium-145 .931 .617 .061 .022 * EC
Tantalum-179 1.82 .110 .065 0 EC
Promethium-145 17.7 131 .164 .072 .022 * EC
Samarium-151 90.0 11.6 .077 .077 * β
Platinum-193 50 17.5 .057 .076 3.81 * EC
Cadmium-109 1.26 .216 .088 .57 * EC
Thulium-171 1.91 606 .096 .096 30.8 * β
Gadolinium-153 .658 .484 .100 * EC
Iron-55 2.74 3140 .231 .126 .36 * EC
Cobalt-57 .744 .836 .136 0 EC
Europium-155 4.76 705 .252 .147 312 * β
Cerium-139 .377 .278 .166 * EC
Gadolinium-153 .658 .484 .173 * EC
Gold-195 .510 .227 .210 EC
Promethium-147 2.6 1200 .224 .224 1.3 * β
Calcium-45 .445 .257 .257 * β
Rhodium-101 3.3 9890 .541 .325 0 EC
Europium-149 .255 .692 .328 0 EC
Barium-133 10.51 758 .517 .384 * EC
Tin-121m 43.9 153 .396 .390 .01 * IT & β
Beryllium-7 .146 .862 .478 0 EC
Vanadium-49 .901 232 .602 ? 0 EC
Making radium from polonium needs extreme neutron flux. The hurdle is polonium-212 with a half life of 249 nanoseconds. Once you have radium, it's easy to get to thorium.
Half life Neutron capture Decay
second barn
Bismuth 209 Stable .0338
Bismuth 210 433000 e-
Bismuth 211 130 alpha
Polonium 210 12000000 .00123 alpha
Polonium 211 .516 alpha
Polonium 212 .000000249 alpha
Polonium 213 .00000365 alpha
Polonium 214 .000164 alpha
Polonium 215 .00178 alpha
Polonium 216 .145 alpha
Polonium 217 1.47 alpha 95%, beta 5%
Polonium 218 186 alpha
Polonium 219 618 beta 71.8%, alpha 28.2%
Polonium 220 40 beta
Polonium 221 132 beta
Polonium 222 546 beta
Polonium 223 6 beta
Polonium 224 180 beta
Polonium 225 10 beta
Polonium 226 60 beta
Polonium 227 2 beta
Astatine 217 .0323 alpha
Astatine 218 1.27 alpha
Astatine 219 56 alpha 97%, beta 3%
Astatine 220 223 beta 92%, alpha 8%
Astatine 221 138 beta
Astatine 222 54 beta
Astatine 223 50 beta
Astatine 224 150 beta
Astatine 225 3 beta
Astatine 226 420 beta
Astatine 227 5 beta
Astatine 228 60 beta
Astatine 229 1 beta
Radon 225 280 beta
Radon 226 444 beta
Radon 227 20.8 beta
Radon 228 65 beta
Radon 229 12 beta
Radon 230 ? beta
Radon 231 ? beta
Francium 228 38 beta
Francium 229 50.2 beta
Francium 230 19.1 beta
Francium 231 17.6 beta
Francium 232 5 beta
Francium 233 .9 beta
Radium 223 988000 alpha
Radium 224 314000 alpha
Radium 225 1290000 beta
Radium 226 1600 year alpha
Radium 227 2550 beta
Radium 228 182000000 beta
Radium 229 240 beta
Radium 230 5580 beta
Radium 231 103 beta
Radium 232 250 beta
Radium 233 30 beta
Radium 234 30 beta
 |
|---|